Understanding a Turf Fertilizer Label
Proper fertilization is critical to maintain lawn health and vigor. While general fertility guidelines can aid in maintaining high quality turfgrass, even the best recommendations are of little value if one cannot accurately apply the fertilizer recommended. In order to intelligently purchase and apply turfgrass fertilizers, one must be able to read and understand a fertilizer label. Many different types of fertilizer are available for use on turfgrass. Complete fertilizers contain nitrogen (N), phosphorous (P), and potassium (K) and are widely used for turfgrass fertilization. For certain situations, incomplete fertilizers containing some combination of N, P, and K but not all three elements, may be the best choice. Every fertilizer material, whether complete or incomplete, must carry a label stating the guaranteed analysis of the material. The exact label information may vary from state to state since no uniform federal regulations exist; however, the manufacturer is usually required to include the following label information:
- Name, brand, or trade mark.
- Guaranteed chemical analysis.
- Potential acidity (CaCO3 equivalent).
- Manufacturer's name and address.
- Net weight of fertilizer in the container.
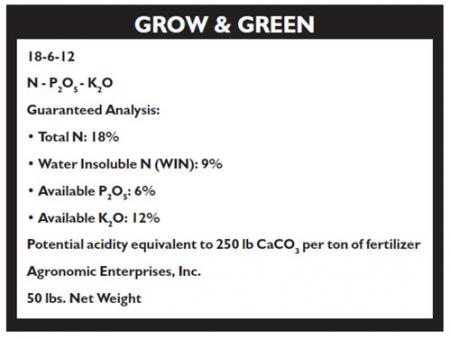
Sample label:
Guaranteed Analysis
The guaranteed analysis (or fertilizer grade) is a listing of nutrients contained in the bag, by weight. The first number of the analysis lists % N, the second number represents % P (as P2O5), and the third number % K (as K2O). The guaranteed analysis of the fertilizer in the above example is: 18-6-12. Occasionally, a fourth number may appear prominently on the label. In turf fertilizers, this fourth number usually represents either iron (Fe) or sulfur (S), two supplemental nutrients that may be of value under special conditions.
Ratio
The ratio of a fertilizer is an important characteristic to understand. The ratio of a fertilizer is the relationship between the N - P2O5 - and K2O content of a fertilizer. The ratio of the fertilizer in the example above is 3-1-2. A fertilizer with a 3-1-2 ratio contains one and a half times as much N as K2O and three times more N than P2O5. Fertilizer grades of 45-15-30, 36-12-24, and 9-3-6 also have a 3-1-2 ratio. Pound for pound, a 36-12-24 fertilizer contains twice the nutrients of a 18-6-12 fertilizer. Thus, to supply equal amounts of nutrients, one could use 1/2 as much 36-12-24 as 18-6-12 to obtain a similar response.
Actual N, P and K
The fertilizer ratio does not usually reflect N, P, and K content. Only N is expressed on an actual elemental basis. P and K are expressed on an oxide basis; that is, they are contained in the phosphate and potash compounds. Thus, an 18-6-12 analysis fertilizer contains:
- 18% N
- 6% P2O5
- 12% K2O
100 lbs. of 18-6-12 would contain 18 lbs. N, 6 lbs. P2O5 and 12 lbs. K2O.
A 50 lb. bag of 18-6-12 contains:
- 9 lbs. N
- 3 lbs. P2O5
- 6 lbs. K2O
P2O5 contains 44% actual P. In order to find how much actual phosphorous (P) is in the above example (a 50-lb. bag of 18-6-12) multiply 3 lbs. P2O5 by 0.44 (44% of P2O5 is actual P):
(3 lbs. P2O5) (0.44) = 1.3 lbs. actual P.
K2O contains 83% actual K. In order to find how much actual potassium (K) is in the above example (a 50-lb. bag of 18-6-12), multiply 6 lbs. K2O by 0.83 (83% of K2O is actual K):
(6 lbs. K2O) (0.83) = 5 lbs. actual K.
Therefore, 50 lbs. of 18-6-12 contains these actual nutrients:
- 9 lbs Nitrogen (N)
- 1.3 lbs. Phosphorus (P)
- 5 lbs. Potassium (K)
If possible, a soil test should be performed to determine the ratio and amount of phosphate and potash which should be applied. If P and K levels are adequate, (> 10 ppm P, > 100 ppm K), an incomplete fertilizer containing only N would be a good choice. If a soil test is not immediately available, a fertilizer ratio of about 3-1-2 to 5-1-2 is normally recommended for turf. It is not necessary that the ratio be exactly 3-1-2 or 5-1-2 if it is close to that ratio. For late spring, late summer and early fall fertilization, a fertilizer with a balanced ratio of N and K (that is, one part nitrogen for every one part potassium) is recommended to increase turfgrass tolerance to heat, drought, cold, and stress.
Water Insoluble Nitrogen (WIN)
In addition to listing % N, P2O5, and K2O by weight, the label of a turf fertilizer further describes how much of the total N is water insoluble nitrogen (WIN). WIN is nitrogen which is slowly released for use by the turf over a long period of time (several weeks, months or years) as opposed to quickly available water soluble nitrogen (WSN).
For lawn maintenance, a fertilizer containing both WSN and WIN is desirable. WSN is quickly available to the turf and thus provides improved color and growth very soon after application. However, it is more likely to cause foliar burn at rates exceeding 2 lbs. of N/1000 sq. ft. or during periods of hot, dry weather. At normal rates of 1 lb. of N/1000 sq. ft., the response from WSN will last approximately 4-6 weeks depending upon climatic conditions. If a longer period of response (and thus fewer applications) is desired, a fertilizer containing some of the N as WIN should be considered.
WIN is slowly released by one of several mechanisms and, therefore, is less likely to cause foliar burn; it provides a longer lasting response than quickly available WSN. It is also more expensive per pound of N applied, especially if the WIN is derived from natural organic sources such as sewage sludge, plant extracts, proteins, etc. The guaranteed analysis shown in the example lists 18% total nitrogen and 9% WIN. Thus, 50% of total nitrogen (9%/18% or 0.09/0.18) is in a slowly available WIN form. This will provide acceptable, near term response while extending the period between fertilizations.
Calculating How Much Fertilizer to Apply
In order to determine how much fertilizer to apply to an area, one must know:
- square footage of turf to be treated.
- recommended application rate.
- the analysis of the fertilizer.
Example: (click image to show larger image)
 Grow & Green fertilizer (18-6-12) has been chosen to provide 1 lb. of total N per 1000 sq. ft. of area. The turf area is 7,000 sq. ft. How much Grow & Green fertilizer is needed to fertilize the area?
Grow & Green fertilizer (18-6-12) has been chosen to provide 1 lb. of total N per 1000 sq. ft. of area. The turf area is 7,000 sq. ft. How much Grow & Green fertilizer is needed to fertilize the area?
This same calculation can be used to determine how much of any fertilizer to purchase to supply any nutrient if you know the square footage to be treated, the fertilizer analysis, and the recommended rate of the nutrient to be applied.
CaCO3 Equivalent
Most fertilizers, especially complete fertilizers, tend to cause an acidic reaction in soils. The acidification is often a result of the oxidation of the ammonium (NH4+) which provides the fertilizer's N. Phosphorous and potash fertilizers commonly have little effect on pH unless they also contain nitrogen. The CaCO3 equivalent is a measure of the acidifying potential of a fertilizer. It expresses how much CaCO3 (calcium carbonate; limestone) would have to be applied to the turf area to counteract the acidifying effects after one ton (2000 lbs.) of the fertilizer had been applied to the area. The acidifying nature of a fertilizer is rarely of critical concern, but in the absence of a soil test, is a way of partially estimating liming requirements over a long period of time.
See sample label. The Grow & Green fertilizer has a potential acidity equivalent to 250 lbs. CaCO3 per ton of fertilizer.
Example: (click image to show larger image)
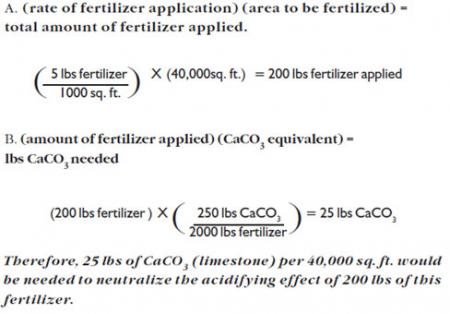 Grow & Green fertilizer is applied at a rate of 5 lbs./1000 sq. ft. to a lawn. How much CaCO3 (limestone) would have to be applied to counteract the effect of 5 lbs. fertilizer/1000 sq. ft. applied to a 40,000 sq. ft. area?
Grow & Green fertilizer is applied at a rate of 5 lbs./1000 sq. ft. to a lawn. How much CaCO3 (limestone) would have to be applied to counteract the effect of 5 lbs. fertilizer/1000 sq. ft. applied to a 40,000 sq. ft. area?
Revised: 05/2011