Soil Health & Nutrient Management
Soil Health
What is Soil Health?
Soil health (or soil quality) has been defined as the capacity of a soil to sustain plant and animal productivity, maintain or enhance water and air quality, and support human health and habitation over a human time scale. In more specific terms, a healthy soil must have: good tilth and drainage, sufficient depth for crop growth, sufficient exchangeable nutrient supply (not excessive or prone to leaching), small population of weeds, insect pests or plant pathogens, large population of beneficial organisms, no toxins, and resilience to adverse conditions. A number of individual soil tests may be used to assess soil health (including those obtained with Routine Soil Analysis); however, a comprehensive evaluation should include a suite of complementary tests to measure soil chemical, physical, and biological properties.
Characteristics of Healthy Soils
(summarized from Cornell's Berry Soil and Nutrient Management – A Guide for Educators and Growers)
What are the characteristics of a healthy soil? Sufficient soil depth for plant root development is important; a soil depth of 8 inches or greater is preferred in the case of berry crops. A healthy soil should have good tilth, water storage and drainage. It should have sufficient but not excessive nutrients and be free of chemicals harmful to plants such as heavy metals, herbicide residues or other contaminants.
Healthy soils should have low populations of plant disease and parasitic organisms such as fungi, bacteria, nematodes, springtails, and so on. Conversely, a healthy soil should contain high populations of beneficial organisms like mycorrhizae and earthworms.
Finally, healthy soils should exhibit resistance to being degraded and along with that – resiliency or the ability to recover quickly from adverse events such as flooding, drought, hurricanes, etc.
Understanding the Three Soil Health Processes
 Think of soil health then in terms of the three major realms that impact it: the physical, the chemical, and the biological. These three realms intercept and interact (Figure 2). If any process is compromised, the others are also affected. A healthy soil is balanced in this respect and therefore provides for better growing conditions, crop resiliency and reduced inputs.
Think of soil health then in terms of the three major realms that impact it: the physical, the chemical, and the biological. These three realms intercept and interact (Figure 2). If any process is compromised, the others are also affected. A healthy soil is balanced in this respect and therefore provides for better growing conditions, crop resiliency and reduced inputs.
Over past decades, chemical aspects of soil were, in general, perhaps overemphasized. While good testing procedures and crop recommendations resulted from this focus, not nearly as much attention was paid to the physical and biological aspects of soil. Research is ongoing in the physical and biological realms today, providing a more complete understanding of soil health and as a result, more comprehensive short-term and long-term management strategies for soil health improvement.
The Chemical Processes
The chemical processes in soil provide essential nutrients for plants. Soil pH is a critical component of the chemical process as it affects nutrient availability. Any changes in soil pH must be addressed, before the planting is established; failure to adjust soil pH to optimal levels for the crop will seriously impact plant establishment as well as future crop production. Soil pH adjustment is more difficult after a perennial crop is established and may reduce the success of the planting.
Chemical processes also includes both macronutrients (nutrients needed in larger quantities, such as N, P and K), secondary nutrients like Ca, Mg and S, and micronutrients required in smaller quantities (such as B and Zn); specific recommendations have been developed for correcting deficiencies of these nutrients essential for berry crop production.
The Physical Processes (summarized)
The physical processes of soil may be limited by inherent or dynamic qualities; some of these may be remediated; others may not.
- Internal drainage – poor internal drainage reduces root growth and function and may support disease development.
- Water availability is a function of soil texture, soil organic matter content, and rooting depth.
- Soil aggregate (crumbs) stability – is a function of adequate soil organic matter which generates humates and other substances that hold soil particles together and contribute to good soil tilth.
- Soil structure – soils with a range of pore sizes are able to provide good drainage, aeration, and rooting, while also retaining moisture.
- Compaction – compaction layers, either near the surface or deeper, can inhibit root penetration and also water drainage contributing to excess runoff or erosion.
The Biological Processes
Understanding soil biology is very much at forefront of our science today. Soil represents a complex environment with highly variable conditions. Most biological activity occurs near the surface of the soil where most of the organic matter is located. There are 3 general types of organic matter found in soil: Living, dead, and very dead. All 3 play important roles in helping produce high yields of healthy crops. Adding organic matter to soil results in many benefits.
- Living Organic Matter - includes plant roots, bacteria, fungi, nematodes, and many other types of organisms. They use resources in soil in various ways, decomposing organic matter, cycling nutrients to make them available for plants, influencing other biota (such as by supressing pathogens), and responding to their chemical and physical environment in very complex ways.
- Dead Organic Matter - is composed of recently dead soil organisms and crop residues that provide food (energy and nutrients) for soil organisms to live and function. Dead organic matter is also called “active” or “particulate” organic matter. This is the other essential partner in mineralizing nutrients for plants, aggregating soils, and forming humus.
- Very Dead Organic Matter - is not a biologically active fraction; rather it consists of well-decomposed organic materials, also called humus. Humus supports the chemical activities of soil; it contains very high amounts of negative charges that hold nutrients and cations in the soil. Humus also has high water-holding capacity, and stores carbon.
Plant Nutrients - Macro & Micro
Nitrogen
Nitrogen (N) greatly influence the growth and yield of crops. Management of soil and fertilizer N is difficult because N undergoes numerous transformations and is easily lost from the soil. These losses concern growers for three principal reasons: 1) N losses can and often do adversely affect plant growth and crop yield, 2) when N is lost in the nitrate form, there is a chance for contamination of groundwater and drinking water supplies, and 3) it is expensive to replace lost N.
The Nitrogen Cycle
This Nitrogen Cycle illustration shows nitrogen (N) inputs, losses and transformations. 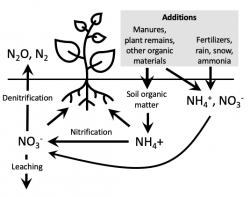 When inputs exceed plant needs, nitrates can accumulate in the soil and pose a threat to groundwater. Conversely, when plant-available forms of N from the soil and any inputs are too low, crop growth suffers. The key to successful management of N is to find the relatively "thin line" between too much and too little N. It is not an easy task. N transformations and losses are affected by soil conditions and the vagaries of the weather. The rates of most N inputs are difficult to accurately estimate.
When inputs exceed plant needs, nitrates can accumulate in the soil and pose a threat to groundwater. Conversely, when plant-available forms of N from the soil and any inputs are too low, crop growth suffers. The key to successful management of N is to find the relatively "thin line" between too much and too little N. It is not an easy task. N transformations and losses are affected by soil conditions and the vagaries of the weather. The rates of most N inputs are difficult to accurately estimate.
Nitrogen Inputs
As can be seen from the N cycle, there are two sources of the N used by plants: ammonium (NH4) and nitrate (NO3). In addition to commercial fertilizer sources, available N may be added to the soil through mineralization (the microbial conversion of organic N to ammonium and then nitrate) of soil organic matter, manure and other organic residuals, and plant litter.
Soil organic matter: Organic matter contains the largest pool of soil N, usually comprising more than 90 percent of total soil N. The total amount of N in the plow layer of agricultural soils is surprisingly large. One can estimate the total N in pounds per acre in the 6" to 7" of surface soil by multiplying the soil's organic matter content by 1,000. Thus, a soil with 4% organic matter contains about 4,000 lbs total N per acre.
The amount of this total N available to plants in any one year, however, is relatively small. Research has shown that for most soils 2 to 4% of the total N is converted (mineralized) annually to forms plants can use. Thus, soil with a total of 4,000 lbs N per acre would produce 80 to 160 lbs N per acre annually for plant use. If the crop needs 200 lbs N per acre for adequate growth and development, some additional N must come from non-soil sources. Manure and/or fertilizer are the most likely candidates to furnish rapidly available N. The rate of mineralization is dependent on microbial activity, especially bacterial activity. Such activity is favored by warm soils with adequate, but not excessive moisture and a pH above 6. These conditions are also favorable to most fruit crops. On well-managed soils used for fruit production, 20 to 40 lbs of N per acre will become available during the growing season for each percent of organic matter if the weather is favorable.
Manures and other waste products: The N content of manures and their N fertilizer equivalents are highly variable. Differences in N content are due to the species of animal, the animal's age and diet, the moisture content of the manure, handling and storage and the amount of bedding in the manure. The N fertilizer equivalent of a given manure varies not only with the animal species and the total N content of the manure, but also with the time of application (Table 3). The values in this table are based on numerous analyses of Connecticut manures as well as published data from other states. If specific manure analysis data for the farm are not available, growers should estimate N credits by the table. The time elapsed between spreading and incorporation is also important. About half of the N in dairy manure and three quarters of the N in poultry manure is in the form of ammonia, which is volatile. If left on the soil surface, this N will volatilize and be lost. To avoid this loss, manure should be incorporated shortly after spreading. NOTE: Manure has the potential to contain human pathogens. Various application practices can be employed to limit the risk of pathogens from the manure ending up on fruit (see the Produce Safety section for management practices).
Previous manure applications: Up to 50% of the total N in cow manure is available to crops in the year of application. Between 5% and 10% of the total applied is released the year after the manure is added. Smaller amounts are furnished in subsequent years. The quantity of N released the year after a single application of 20 tons per acre of cow manure is small (about 15 lbs N per acre). However, in cases where manure has been applied at high rates (30 to 40 tons per acre) for several years, the N furnished from previous manure increases substantially.
The buildup of a soil's N-supplying capacity resulting from previous applications of cow manure has important consequences for efficient N management, two of which are:
- The amount of fertilizer N needed for the crop decreases annually;
- If all the crop's N needs are being supplied by manure, the rate of manure needed decreases yearly.
With cage layer poultry manure, a higher percentage of the total N in the manure is converted to plant-available forms in the year of application. Consequently, there is relatively less carry-over of N to crops in succeeding years. This is due to the nature of the organic N compounds in poultry manure. This does not mean, however, that there is never any carry-over of N from poultry manure applications. If excessive rates of poultry manure (or commercial N fertilizers) are used, high levels of residual inorganic N, including nitrate, may be in the soil the following spring. High levels of soil nitrate in the fall, winter and spring have the potential to pollute groundwater and coastal sea water.
Previous crops: Cover crops can supply appreciable amounts of N to succeeding crops. Legumes, such as alfalfa and red clover, can provide 100 pounds or more of N to crops that follow. Other legumes, mixed grass-legume stands and grass sods supply less N to succeeding crops (Table 2). Keep in mind that most of the N is in the leaves, not the roots. If a legume hay crop is harvested, most of the N is removed from the field along with the hay.
| Previous Crop | Nitrogen Credit Lbs N per acre |
|---|---|
| Grass sod | 20 - 40 |
| “Fair” clover (20-60% stand) | 40 - 60 |
| “Good” clover (60-100% stand) | 60 - 90 |
| “Fair” alfalfa (20-60% stand) | 60 - 90 |
| “Good” alfalfa (60-100% stand) | 100 - 150 |
| Sweet corn stalks | 30 |
| “Good” hairy vetch winter cover crop | 120 - 150 |
Compost as a nutrient source: Finished compost is a dilute fertilizer, typically having an analysis of about 1-1-1 (N-P2O5-K2O). The nitrogen content of composts varies according to the source material and how it is composted. In general, nitrogen becomes less available as the compost matures. Nitrogen in the form of ammonium (NH4+) or nitrate (NO3-) is readily available, however in a finished compost there should be little ammonium, and any nitrate that is produced could have leached away, especially if the compost is cured or left out in the open. The majority of the nitrogen in finished compost (usually over 90%) has been incorporated into organic compounds that are resistant to decomposition. Rough estimates are that only 5% to 15% of the nitrogen in these organic compounds will become available in one growing season. The rest of the nitrogen will become available in subsequent years. NOTE: Compost made with animal source material has the potential to contain human pathogens. Compost management and application practices can be employed to limit the risk of contaminated compost ending up on fruit (see Produce Safety for management practices).
Synthetic fertilizers: Fertilizers used to supply N include urea (46-0-0), diammonium phosphate (DAP: 18-46-0), monoammonium phosphate (MAP: 11-48-0), urea-ammonium nitrate solution (UAN: 32-0-0), calcium ammonium nitrate, calcium nitrate, potassium nitrate and various manufactured and blended fertilizers such as 15-8-12, 15-15-15 and 10-10-10. In bulk blended or custom blended mixes, N-containing fertilizers with almost any grade can be provided.
| APPLICATION TIMING (lbs N/ton) | |||
|---|---|---|---|
| Kind of Manure | April/May1 | Fall Only2 | Other times3 |
| DAIRY (COW) | |||
| Solid | 5 | 2 | 3 |
| Liquid | 16 | 18 | 12 |
| POULTRY, CAGE LAYER | |||
| fresh (20-40% DM)4 |
16 | 5 | 8 |
| sticky-crumbly (41-60% DM) |
22 | 7 | 11 |
| crumbly-dry (61-85% DM) |
32 | 10 | 16 |
|
1 “April/May” credits refer to manure applied and incorporated in April and/or May for spring-planted crops and for manure applied and incorporated within four weeks of planting at times other than spring. 2 Use “fall only” values for manure applied in no-till or maintenance situations where the manure is not incorporated. 3 "Other times” means any time or any combination of times other than April/May or fall only for manure applied for spring-planted crops. 4 DM = Dry matter. |
|||
Nitrogen Losses
Nitrogen losses occur in several ways. The loss of available soil N not only costs growers money, it has the potential to negatively impact both air and water quality. Understanding the cause of N losses can help growers make management decisions to improve N use efficiency and minimize negative environmental impact.
Volatilization Losses: These losses occur mainly from surface-applied manures and urea. The losses can be substantial — more than 30% of the N in top-dressed urea can be volatilized if there is no rain or incorporation within two or three days of application. Losses are greatest on warm breezy days. Volatilization losses tend to be greater from sandy soils with pH values above 7. Incorporate manures right after applying them to avoid volatilization losses. Under the right conditions more than 50% of the ammonium N may be volatilized within the first 48 hrs. of applying manure if it is not incorporated.
Not only does volatilization reduce the fertilizer value of manure and urea, it can degrade air and water quality. Ammonia in the atmosphere can form particulates that contribute to smog. Ammonia emissions can also contribute to eutrophication of surface waters via atmospheric deposition.
Leaching Losses: Nitrogen can be lost by leaching in either the ammonium or nitrate form. Usually, much more N is leached as nitrate than as ammonium. Leaching losses are greatest on permeable, well- or excessively-drained soils underlain by sand or gravel when water percolates through the soil. Percolation rates are generally highest when the soil surface is not frozen and evapotranspiration rates are low. Thus, late fall and early spring are times when leaching potential is greatest. Cover crops growing during these times can take up this residual N and prevent it from leaching. The N will then be released for crop use after the cover crop is plowed down in the spring. Of course, leaching can occur any time there is sufficient rainfall or irrigation to saturate the soil. This is why it is important to attempt to match fertilizer N application rates with crop N needs.
Denitrification Losses: These losses occur when nitrate is converted to gases such as nitrous oxide (N2O) and nitrogen (N2), when the soil becomes saturated with water. Poorly drained soils are particularly susceptible to such losses. In especially wet years on some soils, more than half the fertilizer N applied can be lost through denitrification. Favorable conditions for denitrification often occur in early spring and late fall. Minimizing the concentration of nitrate in the soil during these periods by delaying N application in the spring and planting cover crops in the fall will help reduce denitrification losses.
Immobilization: Immobilization occurs when soil micro-organisms absorb plant-available forms of N. The N is not really lost from the soil because it is held in the bodies of the microorganisms. Eventually, this N will be converted back to plant-available forms. In the meantime, however, plants are deprived of this N, and N shortages in the plants may develop. Immobilization takes place when highly carbonaceous materials such as straw, sawdust or wood chips are incorporated into the soil. Manure with large amounts of bedding may cause some immobilization.
Crop Removal of Nitrogen: In most cases, the greatest removal of N from the soil is via crop removal. Strawberries remove approximately 100 lbs of N per acre annually (in foliage and harvested fruit). On the other hand, mature highbush blueberries only remove approximately 50-60 lbs of N per acre (in foliage, wood and harvested fruit). Raspberries likely remove somewhere in between. Anticipated crop removal of N is one of the factors used in calculating N budgets and making N fertilizer recommendations. Depending on the crop, variable amounts of the N absorbed by the crop are returned to the soil after harvest in non-harvested plant parts. For example, strawberry renovation returns a significant amount of crop biomass plus straw mulch to the soil, contributing to the N budget.
Phosphorus
Phosphorus (P) is referred to as P2O5 (phosphate) for the purposes of soil testing, fertilizer grades and recommendations. Among other important functions, phosphorus provides plants with a means of using the energy harnessed by photosynthesis to drive its metabolism. Deficiency can lead to impaired vegetative growth, weak root systems, poor fruit and seed quality, and low yield; however, excessive soil phosphorus levels are a concern due to the potential negative impact on surface water quality. Most P losses occur with runoff, but where soil levels are extremely high, subsurface losses can occur. Phosphorus enrichment is a leading source of water quality impairment of many lakes, streams, and rivers in New England.
Soil P exists in a wide range of forms. Some P is present as part of soil organic matter and becomes available to plants as the organic matter decomposes. Most inorganic soil P is bound tightly to the surface of soil minerals (e.g.., iron and aluminum oxides). Warm, moist, well-aerated soils at a pH level of about 6.5 optimize the release of both of these forms. Soil tests attempt to assess the ability of soil to supply P from bound forms during the growing season. When a soil test indicates that P is low and fertilizer is needed, the rate recommended is intended to satisfy immediate crop needs and begin to build soil P levels to the optimum range (i.e., build and maintain). Once soil test levels are in the optimum range, only a small amount of P is needed to replace the amount removed each year to maintain soil levels.
If your soil test results indicate above optimum levels, P application is unnecessary and should be limited. Where soil P levels are excessive, P application should be eliminated, and additional steps should be taken to minimize the risk of surface water contamination by limiting runoff losses.
Potassium
Potassium (K) is expressed as K2O similar to the way P is expressed as P2O5. Crop need for K varies. Plants use K to open and close stomates and to move nitrates from the roots to the leaves. Potassium rivals N as the nutrient absorbed in greatest amounts by plants. Like N, crops take up a relatively large proportion of plant-available K each growing season. Plants deficient in K are unable to utilize N and water efficiently and are more susceptible to disease. Most available K exists as an exchangeable cation (see below). The slow release of K from native soil minerals and from fixed forms in clays can replenish some of the potassium lost by crop removal and leaching. This ability, however, is limited and variable. Fertilization is often necessary to maintain optimum yields. See the table at the beginning of each crop section for the potassium needs for each crop.
It is important that the soil K plus the applied K is enough to meet crop needs. However, excessive levels should be avoided because K can interfere with the uptake of Ca and Mg (see “Base Saturation”). K is subject to leaching on sandy soils low in organic matter. If high amounts of K are needed, split applications should be used. Potassium sulfate (0-0-50) or sulfate of potash magnesium (Sul-Po-Mag, 0-0-22) are the best sources of potassium for brambles and strawberries. Although muriate of potash (KCl, 0-0-60) is less expensive, brambles are sensitive to the chloride in this fertilizer.
Calcium
Calcium is usually supplied in sufficient quantities by liming if appropriate liming materials are chosen (see “Soil pH and Exchangeable Acidity”). If soil pH is high and Ca is needed, small amounts can be applied as calcium nitrate fertilizer (15% N, 19% Ca). Ca can also be applied without affecting pH by applying calcium sulfate (gypsum, 22% Ca) or superphosphate (14 to 20% Ca).
Magnesium
Magnesium is necessary for chlorophyll production and nitrogen metabolism. High soil potassium levels can lead to reduced uptake of magnesium. Magnesium deficiency is characterized by interveinal reddening on older leaves, beginning at the leaf margin. Magnesium, K, Ca, and P can be applied in late fall after plants are dormant. Nutrients can then move into the root zone and be available when growth begins again in the spring. Magnesium is most economically applied as dolomitic or high-mag limestone (see “Soil pH and Exchangeable Acidity”). If liming is not needed, Sul-Po-Mag (11% Mg, 22% K) can be used. You can order blended fertilizer containing Mg.
Minor Elements
Minor elements are difficult to analyze accurately with soil tests. Plant tissue analyses are more reliable for determining whether or not plants are getting sufficient quantities of minor elements. Of the minor elements, boron (B) and zinc (Zn) are the most likely to be needed to supplement soil levels.
Special Note on Lead
Many laboratories routinely screen all soil samples for elevated levels of extractable lead. Lead is naturally present in most New England soils at low concentrations (15-40 ppm total lead). At these levels, lead generally is thought to present minimal danger to people or plants. Soil pollution with lead-based paint and the tetraethyl lead of past automotive fuels have increased soil lead levels to several thousand ppm in some places. Unless the estimated total lead level in your soil exceeds 299 ppm (Modified Morgan extractable level of 22 ppm) it is simply reported as low and can be considered safe (assuming the sample submitted is representative of the area of concern). Estimated total lead levels above 300 ppm are a concern. In such cases, consult your state's Extension Service for further assistance or see: "Soil Lead: Testing, Interpretation, & Recommendations" for more information on soil lead testing and recommendations.
| CROP | AGE | AMOUNT/TIMINGS (ACTUAL N) | N SOURCE | COMMENTS |
|---|---|---|---|---|
| Strawberries | 0 | 30 lb/A early June 30 lb/A early Sept. |
ammonium or calcium nitrate | Be sure plants are growing well prior to application |
| 1+ | 70 lb/A at renovation 30 lb/A early Sept. |
ammonium or calcium nitrate or urea | Adjust fall amount based on leaf tissue analysis | |
| Raspberries and Blackberries (summer bearing) |
0 | 25-35 lb/A 4 weeks after planting | calcium nitrate | Avoid touching plants with fertilizer |
| 1 | 35-55 lb/A split between May and June | ammonium nitrate or urea | Use higher amount on sandier soils or if irrigation is used | |
| 2+ | 40-80 lb/A split between May and June | |||
| Raspberries (fall bearing) |
0 | 25 lb/A 4 weeks after planting | calcium nitrate | Avoid touching plants with fertilizer |
| 1 | 50-80 lb/A split between May and June | ammonium nitrate or urea |
Use higher amount on sandier soils or if irrigation is used. Adjust based on leaf tissue test on mature plantings |
|
| 2+ | 70-100 lb/A split between May and June | |||
| Blueberries | 0 | Do not fertilize newly planted blueberries |
Soil pH should be adjusted to 4.5-5.0 prior to planting Use ammonium sulfate where soil pH is >5.0 Adjust based on leaf tissue test on mature plantings |
|
| 1 | 15 lb/A, split between May and June | ammonium sulfate or urea DO NOT use aluminum sulfate |
||
| 2 | 20 lb/A, split between May and June | |||
| 3 | 25 lb/A, split between May and June | |||
| 4 | 35 lb/A, split between May and June | |||
| 5 | 45 lb/A, split between May and June | |||
| 6 | 55 lb/A, split between May and June | |||
| 7+ | 65 lb/A, split between May and June | |||
| Currants and Gooseberries | 0 | 25 lb/A, 4 weeks after planting | calcium nitrate | |
| 1 | 50-80 lb/A, split between May, June, August | calcium nitrate | ||
| 2+ | 70-100 lb/A, split between May and early August | calcium nitrate | ||
| Elderberries | 0 | Do not fertilize newly planted elderberries | ||
| 1+ |
Apply 1/8 lb of ammonium nitrate for each year of the plant's age, up to one pound per plant |
ammonium nitrate or 10-10-10 |
In spring, spread fertilizer with a spreader in bands one foot wide along both sides of the rows. | |
| Juneberries | 0 | 25 lb/A, 4 weeks after planting | calcium nitrate | Avoid touching plant with fertilizer after planting. |
| 1 | 50-80 lb/A, split between May and June | urea or ammonium nitrate | Use higher amount of sandier soils or if irrigation is used. | |
| 2+ | 70-100 lb/A, split between May and June | urea or ammonium nitrate | Use higher amount on sandier soils or if irrigation is used. Adjust with leaf tissue analysis. |
|
| Source: 2016 Cornell Pest Management Guidelines for Berry Crops | ||||
Soil Testing
Soil & Tissue Testing
Soil tests provide the best way to determine pre-plant requirements for lime and fertilizers. For perennial fruit crops, leaf tissue or petiole analysis are the best ways to determine nutrient status for established crops. With the information from these tests, growers can make informed decisions about fertilizing and liming small fruit crops to achieve optimum yield and quality and to safeguard water quality in a cost-effective manner. Following is a list of soil test laboratories in New England. It is best to use local labs because they are calibrated for local soils and recommendations are tailored to New England conditions.
Soil Testing Labs of New England
soil testing (1), leaf tissue analysis (2), compost testing (3), manure testing (4)
CONNECTICUT
UConn Soil Nutrient Analysis Lab (1, 2)
6 Sherman Place, Unit 5102
Storrs, CT 06269-5102
Telephone: 860-486-4274
Email: soiltest@uconn.edu Website: http://www.soiltest.uconn.edu/
The Connecticut Agricultural Experiment Station (1)
Gregory Bugbee, State Laboratory
123 Huntington St., P.O. Box 1106
New Haven, CT 06504
Telephone: 203-974-8521
Email: Gregory.Bugbee@ct.gov Website: https://portal.ct.gov/CAES/Soil-Office/Soil-Office/Soil-Testing-Offices-Instructions
MAINE
The Analytical Laboratory and Maine Soil Testing Services (1,2,3,4)
5722 Deering Hall, Room 407
Orono ME 04460-5722
Telephone: 207-581-3591
Email: hoskins@maine.edu Website: https://umaine.edu/soiltestinglab/
MASSACHUSETTS
UMass Soil & Plant Tissue Testing Laboratory (1,2)
203 Paige Laboratory/UMass
161 Holdsworth Way
Amherst MA 01003-9302
Telephone: 413-545-2311
Email: soiltest@umass.edu Website: https://ag.umass.edu/soiltest
UNH Cooperative Extension Soil Testing Program (1,2,3)
Barton Hall B206
Durham NH 03824
Telephone: 603-862-3200
Email: soil.testing@unh.edu Website: https://extension.unh.edu/agriculture-gardens/pest-disease-growing-tools/soil-testing-services
VERMONT
UVM Agricultural & Environmental Testing Lab (1,3,4)
262 Jeffords Hall, 63 Carrigan Drive, UVM
Burlington VT 05405
Telephone: 802-656-3030, 800-244-6402
Email: AgTesting@uvm.edu Website: https://www.uvm.edu/extension/agricultural-and-environmental-testing-lab
http://www.blinc.com/agriculture-analysis
Woods End Research Lab, Inc. (1,3)
290 Belgrade Rd., P.O. Box 297
Mt. Vernon, ME 04352
Telephone: 207-293-2457
Email: lab@woodsend.com Website: https://woodsend.com/
1087 Jamison Rd.
Washington Court House, OH 43160
Telephone: 800-321-1562
http://www.spectrumanalytic.com/
Taking a Soil Sample
Although soil samples can be taken any time, many prefer to take samples in summer or fall because this allows time to apply any needed lime, plan a fertility program and order materials well in advance of spring planting. Avoid sampling when the soil is very wet or soon after a lime or fertilizer application. If a field is uniform, a single composite sample is sufficient. A composite sample consists of 10 to 20 sub-samples taken from around the field and mixed together. To obtain sub-samples, use a spade or a soil probe to take cores or thin slices of soil representing the top 6” to 8” of soil. Put these sub-samples into a clean container and thoroughly mix. Take about one cup of the mixture, dry it at room temperature, put it in a zip lock bag and tightly close it. Label each sample on the outside of the bag. To get accurate recommendations, make sure to download and fill out sample submission forms from the lab where samples will be sent.
In many cases, fields are not uniform, due to uneven topography, wet and dry areas, different soil types, or areas with varying previous crop and fertilizing practices. In such cases, the field should be subdivided and composite samples tested for each section.
Soil testing laboratories vary somewhat in their services and prices. Soils should be tested for organic matter content every two or three years. Be sure to request this if it is not part of the standard test. For more information, check with your state’s laboratory or Extension Specialist.
Cation Exchange Capacity
Cation exchange capacity (CEC) is a measure of the soil’s ability to retain and supply nutrients, specifically the positively charged nutrient ions called cations. These include the cations calcium (Ca2+), magnesium (Mg2+), potassium (K+), ammonium (NH4+), and many of the micronutrients. Cations are attracted to negatively charged surfaces of clay and organic particles called colloids. CEC is reported in units of milli-equivalents per 100 grams of soil (meq/100 g) and can range from below 5 meq/100 g in sandy, low organic matter soils to over 15 meq/100 g in finer textured soils and those high in organic matter. Low CEC soils are more susceptible to cation nutrient loss through leaching, and may not be able to hold enough nutrient cations for a whole season of crop production.
Base Saturation
The cations calcium (Ca2+), magnesium (Mg2+), potassium (K+), hydrogen (H+) and aluminum (Al3+) account for the vast majority of cations adsorbed on the soil colloids in New England soils. Hydrogen (H+) and aluminum (Al3+) are considered acidic cations because they tend to lower soil pH while calcium (Ca2+), magnesium (Mg2+), and potassium (K+) are considered basic cations and have no direct influence on soil pH. Base saturation is the portion (expressed as a percentage) of the soil’s cation exchange capacity occupied by calcium (Ca2+), magnesium (Mg2+), and potassium (K+). At one time, many labs provided fertilizer recommendations to achieve very specific ideal potassium, calcium, and magnesium saturation ratios. This approach was never well supported by data. Research conducted over the last several decades indicates that an ideal basic cation ratio does not exist and fertilizing to achieve a prescribed level of potassium, calcium, and magnesium saturation is unjustified. Still, base saturation can provide useful information. Your report will include the base cation saturation values observed for your sample and the ranges typically observed in New England soils. When base saturation is well outside of these ranges it is typically associated with deficient or excessive potassium or very acidic or alkaline soil conditions. Following the fertilizer and lime recommendations provided with your report will typically result in base saturation values within normal ranges.
Soil pH and Exchangeable Acidity
One of the most valuable pieces of information you can get from soil testing is a measure of the soil pH. Soil pH is an indicator of the soil’s acidity which is a primary factor controlling nutrient availability, microbial processes, and plant growth. A pH of 7 is neutral, less than 7 is acidic, and greater than 7 is alkaline. Maintaining proper soil pH is one of the most important aspects of soil fertility management. When the soil is acidic, the availability of nitrogen, phosphorus, and potassium is reduced, and there are usually low amounts of calcium and magnesium in the soil. Under acidic conditions, most micronutrients are more soluble and are therefore more available to plants. Under very acidic conditions aluminum, iron, and manganese may be so soluble they can reach toxic levels. Soil acidity also influences soil microbes. For example, when soil pH is low (below 6), bacterial activity is significantly reduced. Acidic soil conditions also reduce the effectiveness of some herbicides.
When soil pH is maintained at the proper level, plant nutrient availability is optimized, solubility of toxic elements is minimized, and beneficial soil organisms are most active. While most plants grow best in soil with a pH between 6 and 7, there are some notable acid-loving exceptions, such as blueberry, which performs best in soils with a pH near 5.
Due to the climate and geology of New England, soils here tend to be naturally acidic (4.5-5.5).The most effective way to increase soil pH is to apply agricultural limestone. The quantity of lime required is determined by the target pH (based on crops to be grown) and the soils buffering capacity. Buffering capacity refers a soil’s tendency to resist change in pH. Soil pH is a measure of active acidity, based on the concentration of hydrogen ions (H+) in soil solution, and is an indicator of the current soil condition. When lime is added to a soil, active acidity is neutralized by chemical reactions that remove hydrogen ions from the soil solution. However, there are also acidic cations (H+ and Al3+) adsorbed on soil colloids (the CEC) which can be released into the soil solution to replace those neutralized by the lime. This exchangeable acidity, which is reported in units of meq/100 g, is directly related to the quantity of lime required to increase the pH from its current level to the target level determined by the selected crop. Soils such as clays or those high in organic matter have a high cation exchange capacity (CEC) and a potential for large amounts of exchangeable acidity. These soils are said to be well buffered. Buffer pH is a measure of reserve acidity and is used by the soil testing laboratory to estimate buffering capacity and lime requirement. Low buffer pH readings indicate high amounts of reserve acidity, and therefore, high amounts of lime will be recommended.
Occasionally soil pH must be lowered, because either the plant requires acid soil or the soil was previously over-limed. Incorporating elemental sulfur (S) is the most effective way to lower soil pH. Once applied, the sulfur oxidizes to sulfuric acid. Applying 5 to 10 lbs. of sulfur per 1000 sq. ft. will lower the pH of most New England soils by approximately half a unit. Use the lower rate for very sandy soils. No more than 15 lbs. of sulfur per 1000 sq. ft. should be applied at any one time. Retest the soil after 4 to 6 months to determine if more sulfur is needed.
Cover Crops & Green Manures
Cover Crops
Cover crops are grown to protect and/or enrich the soil rather than for short term economic gain. When turned into the soil, a cover crop is called a green manure, so the terms are reasonably interchangeable.
When a cash crop is not growing, it is wise to sow something to protect the soil from wind and water erosion, thus the term cover crop. It is also wise to “rest” your fields by occasionally rotating out of cash crop production, while at the same time growing something to improve soil fertility, thus the term green manure. Some green manure crops can also suppress weeds, by “smothering” them and starving them for light. Use high seeding rates if cover crops are grown for weed suppression.
Depending on their growing requirements, cover crops can be sown after vegetable harvest, between a spring and fall crop or by overseeding into a standing small fruit crop after a final cultivation.
In selecting a green manure crop, consider the following: seed cost, winter hardiness (if applicable), ability to fix nitrogen, suppress weeds, and suitability to soil conditions, tillage equipment and the crop to follow. Here is a list of some common cover crops in New England and a description of their uses.
| Material | Carbon:Nitrogen Ratio |
|---|---|
| Legume hay | 15-19:1 |
| Non-legume hay | 24-41:1 |
| Corn stalks | 42:1 |
| Oat straw | 70:1 |
| Rye straw | 82:1 |
| Cow manure | 18:1 |
| Finished compost | 17-20:1 |
| Agricultural soils | 8-14:1 |
| Hardwood sawdust | 500:1 |
Legumes
Sow when “free” nitrogen is desired for a subsequent cash crop with a high nitrogen demand. Legumes generally require good drainage and fertility. Most grow slowly at first so they do not compete much with weeds until well established. Drill seed for best stands. Mix seed with proper inoculant to insure nodulation. Often sown with a nurse crop such as oats, or in mixes with perennial grasses. When legumes are mowed, tarnished plant bugs may be driven into adjacent crops, such as strawberries or raspberries increasing the likelihood of damage.
Red Clover is a short-lived perennial that is somewhat tolerant of acid or poorly drained soils. Mammoth red clover produces more biomass for plow-down than medium red clover, but does not regrow as well after mowing. Mammoth will often establish better than medium in dry or acid soils. Sow in early spring or late summer.
White Clover is a low-growing perennial, tolerant of shade and slightly acid soil. Ladino types are taller than the Dutch or wild types. White clover is a poor competitor with weeds unless mowed. Suitable for use in walkways or alleys. Expensive seed.
Sweet Clover is a biennial (except for annual types like Hubam) that is deep-rooted and adapted to a wide range of soils. It is a good soil-improving crop with a strong taproot that opens up subsoil. Yellow sweet clover is earlier maturing and somewhat less productive than white sweet clover. Sow in early spring or late summer at 15 to 20 lb/acre. Heavy growth is produced in spring after overwintering. Incorporate in late spring or mid-summer at flowering. May deplete soil of moisture, which can be a problem for subsequent crops in dry years.
Hairy Vetch has become increasingly popular as a cover crop. It can fix tremendous amounts of nitrogen. Generally this cover crop is seeded in the fall after August 15 or before mid September in most areas. It should be allowed to grow at least until mid May before plow down. It is advisable to seed winter rye (30-40 lbs/acre) or oats (40-50 lbs/acre) with the vetch when seeded in the fall to take up unused nitrogen and to ensure a good ground cover for erosion control. Most growers prefer oats to winter rye because the oat will not overwinter and the vetch alone is easier to manage the following spring. Hairy vetch can also be seeded in early spring or summer. When seeded in early April it will produce significant nitrogen in time for a late seeding of sweet corn or brassica. When seeded in the summer it will usually winter kill and the following spring the nitrogen will become available for an early crop. Treat seed with a pea-type inoculant.
Alfalfa requires deep, well-drained soil with a pH near neutral for good growth. It is a long-lived perennial that is probably not worth the expense in a short-term rotation. Fixes large amounts of nitrogen if maintained for several years. Seed early spring or late summer at 15 to 25 lb/acre.
Nonlegumes
These are selected when nitrogen contribution to the soil is not a priority. They tend to grow more rapidly and thus are better at short-term weed suppression than legumes. Late-season grasses are useful for recovering leftover nitrogen after crops have been harvested.
Winter Rye is a common winter cover crop, sown after cash crops are harvested in the fall. It is very hardy, adapted to a wide range of conditions, and seed is inexpensive. The latest-sown cover crop, it produces a lot of biomass in the spring. This adds organic matter to the soil but may be difficult to incorporate prior to crop planting.
Oats are used as a winter cover crop to protect the soil without requiring intensive management in the spring, because they are frost-killed. Shallow incorporation of residues may still be necessary before crop planting. Enough growth is needed before first frost to adequately protect the soil, so plant by late August, at a rate of about 100 lb/acre. Oat residues left on the soil surface may chemically suppress weed growth, and act as a physical barrier. Oats are also a good cover crop to plant any time during the spring or summer when land is out of production. Unlike winter rye, oats grow vigorously and upright when seeded in the spring or summer and compete effectively with weeds. Can grow in soils with low pH (5.5).
Ryegrass is a low-growing cover crop that produces an extensive root system good at capturing leftover nitrogen. It is well suited to undersowing, after last cultivation of a cash crop, in order to establish a winter cover prior to harvest. Annual ryegrass is less expensive than perennial ryegrass, and is more likely to winterkill; however, it may overwinter in milder areas, and perennial ryegrass may winterkill in harsher zones. These crops form a dense sod that reduces erosion.
Sudangrass and Sorghum-sudangrass (Sudex) are fast-growing, warm season crops that require good fertility and moisture to perform well. Under such conditions, their tall, rank growth provides excellent weed suppression. Such heavy growth can be difficult to cut and incorporate. Due to its growth habit, sudan grass should be cut back when growth exceeds 20-25 inches or plowed down if a second growth is not desired.
Buckwheat is a fast-growing summer annual that can be used to protect the soil and suppress weeds for a month or two between spring and fall cash crops. It grows fairly well on acid and low phosphorus soils. It decomposes rapidly and is easy to incorporate, but does not contribute a lot of organic matter to the soil. Mow or incorporate at flowering, prior to setting seed so it does not become a weed in subsequent crops. Grows well in low soil pH. To smother weedy fields, some growers plant two successive crops of buckwheat followed by winter rye. Do not allow buckwheat to go to seed prior to plow-down.
Annual Field Brome: Winter annual grass. Establishes rapidly and has extensive fibrous root system contributing organic matter to soil. Plow down in spring. Seed not readily available so plan ahead.
Japanese Millet: Summer annual grass. Fast growing and competes well with weeds. Establishes faster than sudan grass on cool soils. Can be cut back and allowed to regrow after reaches 20 inches. Can reach 4 ft. in 7-8 weeks. Do not allow to mature and drop seed.
Mustards: This includes white or yellow mustard (Sinapis alba), brown or Indian mustard (Brassica juncea), and black mustard (B. nigra L.). Mustards produce glucosinolates, which are compounds that have broad activity against bacteria, fungi, insects, nematodes and weed seeds. Mustards are often considered “biofumigants”, and managed to try to benefit from these effects by mowing and incorporating into the soil immediately afterwards.
Mixtures
Legumes and grasses are often mixed as cover crops to hedge against failure of one and to get some of the benefits of both. The grass will usually establish quickly, holding soil in place and “nursing” the legume along. By taking available soil N, the grass promotes N-fixation by the legume. Fertilization with N or the absence of mowing favors growth of grass over legume. Some common mixtures, in addition to vetch and rye described above, are red clover and oats (combine or mow oat heads, leaving established clover); ryegrass and white clover for mowed alleys. Timothy is often used as a nurse crop for alfalfa. It is advisable to trial unfamiliar cover crops or mixtures on a small scale to determine if they are suited to your climate and management resources before growing them widely.
Note: N fixed in root nodules moves to the leaves and stems of legumes. If hay is harvested from the field prior to plowing, very little N will be contributed to the subsequent crop.
| Cover Crop | Recommended Seeding Dates | Seeding Rate |
|---|---|---|
| Alfalfa | Early April to late May or Late July to mid August | 14 - 20 lbs/A |
| Buckwheat | Late May to early June or Late July to early August | 60 - 75 lbs/A |
| Clovers (Alsike, Ladino, White) | Early April to late May or Late July to mid August | 4 lbs/A (alsike and white) 2 lbs/A (ladino) |
| Red Clover | Early April to late May or Late July to mid August | 8 - 10 lbs/A |
| Sweet Clover | Early April to mid May or Early August | 12 - 20 lbs/A |
| Hairy Vetch | August to early Sept. | 30 - 40 lbs/A |
| Annual Field Brome | July and August | 20 lbs/A |
| Japanese Millet | Late May to mid July | 20 lbs/A |
| Spring Oats | Early to mid April or Mid August | 100 lbs/A |
| Annual Ryegrass | Early April to early June or Early August to early Sept. | 30 lbs/A |
| Perennial Ryegrass | August to mid Sept. | 25 lbs/A |
| Winter Rye | August to mid Sept. | 80-100 lbs/A |
| Sudan Grass | Late May to Early June | 80 lbs/A |
| Sorghum-Sudan Grass Hybrids | Late May to Early June | 35-50 lbs/A |
| Mustards | Late May to July | 10-25 lbs/A |
Soil Organic Matter
Soil organic matter (SOM) is a small but critical component of soils. SOM is continuously being produced by plants and animals and broken down by soil microbes that use it as a source of energy. As such it provides food for a diverse population of microbes in the soil and this helps prevent any one type of organism, such as a plant pathogen, from dominating. As microbes break down SOM, nutrients are released which are available for plant growth. This process is called mineralization and can provide some or all of the nutrients needed for successful crop production. Soil microbes are most active in warm soils (over 70°F) that are moist, but well aerated, with a pH between 6 and 7 (ideal conditions for most fruit crops). Mineralization of nutrients will proceed rapidly under these conditions.
SOM also improves soil structure. It binds individual soil particles together into aggregates. This makes soil friable, allowing for good drainage, aeration, and root growth. SOM also improves the moisture holding capacity of soils. SOM is also the chief contributor to cation exchange capacity in New England soils.
Adding to Soil Organic Matter
Using compost is an effective way to add organic matter to the soil. Small fruit growers can make compost on the farm although most don’t have enough raw materials to satisfy their needs. Some bring in additional materials such as municipal yard wastes to compost on site. Others purchase compost from the increasing number of commercial composters. Regardless of the source, compost should be finished before use. Finished compost has no recognizable bits of matter and will not heat up after turning. Compost should also be tested for nutrient content. Finished compost should have a low ammonium content, high nitrate level and a pH near neutral. Repeated use of a compost high in a particular element could cause a nutrient imbalance. For more information, obtain a copy of Berry Soil and Nutrient Management – A Guide for Educators and Growers (see Resources in Appendices at the end of this publication).
Animal manure is an excellent source of nutrients and organic matter. About half of the nitrogen in fresh dairy manure and 75% of the nitrogen in poultry manure is in the form of ammonia. Ammonia is subject to loss through volatilization if not incorporated immediately after spreading. In the soil, ammonia is converted to nitrate and is available for plant use. However, nitrate is subject to leaching and large applications should generally be avoided. There are times when readily available N is needed, but fresh manure should be applied only with caution. Many people prefer to compost manure before field application to stabilie the N contained in manure. Manure can be mixed with other materials for composting. Manure samples can be analyzed by several of the laboratories listed under Soil Testing.
Manure often contains human pathogens and therefore application practices that avoid transferring pathogens to produce should be utilized when using manure in food production systems. See more on safe application practices in the Produce Safety section.
Cover crops are used by most growers to protect soil from erosion and to take up unused N. Cover crops also contribute to SOM when they are plowed down, although SOM varies considerably among crops (see Cover Crop section).
Carbon-To-Nitrogen Ratio
Organic matter is broken down by microbes which use carbon (C) for energy. They also have a need for nitrogen. Microbes have a requirement of about one N atom for each 25 C atoms. This is a carbon-to-nitrogen ratio (C:N) of 25:1 or 25. If the organic matter has a higher C:N (more C and less N), microbes will need more nitrogen and will take it from the soil. Microbes are more efficient than crops in obtaining N from the soil. If there is not enough nitrogen for both the microbes and the crop, the crop will not obtain what it needs. Eventually there will be a net gain in nitrogen, but crops can suffer in the short term. If organic matter with a high C:N is applied to soil shortly before planting a crop, additional N may be needed to assure the needs of both the microbe and the crop are met. Organic matter with a C:N of less than 25:1 (25) should not be a problem and in some cases can contribute N for crop use. See Table 5 for examples of C:Ns of some sources of organic matter.
Guidelines for Organic Fertilization
An organic fertility program should consider the biological, physical, and chemical characteristics of the soil in order to optimize and sustain soil fertility and crop production. Organic growers should confer with their certifier to ensure that any amendments are in accordance with USDA National Organic Program (NOP) standards. See Organic Certification for additional information.
Organic Matter
Organic matter management is an essential part of organic agriculture. Generous additions of compost, animal or green manures are needed to feed soil microbes, but organic growers need to carefully monitor soil test P levels when adding organic amendments to the soil (see discussion below Building Soil Organic Matter). Organic matter management is essential because the by-products of decomposition of organic amendments bind soil particles to improve the physical condition, or structure of soil, and also because organic matter is the storehouse of nutrients in the soil. Many nutrients, especially N, P, S, Cu, and Zn, are released when organic matter decomposes. The good structure promoted by organic matter results in enhanced root growth, which increases plant retrieval of soil nutrients, which is a classic synergistic effect. Decaying soil organic matter releases nutrients unevenly during the growing season. In the late spring after the soil warms there is usually a flush of nutrients, and the rate declines after that with the rate during the season dependent mostly on soil moisture. When the release of nutrients, or mineralization, is low, as when soils are cool in the early spring, fertilizing with soluble forms of nutrients may benefit crops. This is why some relatively available phosphorus and nitrogen should be banded, or placed near the roots of crops early in the growing season. For example, use bone meal and dried blood or a seed meal like peanut or soybean to provide some available P and N, respectively, or use a commercial organic fertilizer blend. Information on the nutrient content of various organic soil amendments can be found at MOFGA Fact Sheet #11.
Soil amendments of animal origin (blood meal, manure, compost with animal source material, etc.) have the potential to contain human pathogens. Knowledge of your source material and application practices that avoid the potential of transfer of human pathogens to fruit are an important part of managing your soil (See the Produce Safety section for details).
Nitrogen
Nitrogen is the most common limiting nutrient on organic farms. The most common organic sources of N are seed meals, fish meal, blood meal and livestock manures. Most sources of N used by organic farmers are expensive and that explains why most growers turn to livestock manures, compost or crop rotations with legumes. When using manure or immature compost, remember that only up to half the N becomes available to plants during the season following incorporation. Each ton of compost containing 1% N can provide a crop with 5 to 10 lb of N per acre. When calculating N needs, remember that there is a release of about 20 lb/acre or more of N for each 1% soil organic matter. These releases of N vary with drainage and other soil conditions, and may not be well timed to crop needs, especially early, short season crops. Annual crops need N most intensely about three to four weeks after emergence or transplanting. Therefore, sidedressing, or spreading soluble N along the crop row, at this time is most efficient. Because soluble organic N fertilizers are expensive, it is advisable to use lower rates than recommended for synthetic fertilizers. A sidedressing of 25 lb/acre of actual N is reasonable for many crops growing in a fairly fertile soil. This requires 200 lb dried blood, 400 lb soy or cottonseed meal, or the equivalent from other sources of N.
Phosphorus
Phosphorus is low in many unamended New England soils, and can limit crop growth, especially early in the season. Soils testing less than 10 lb/acre available phosphate (P2O5) usually require substantial applications of phosphate. Hard rock phosphate contains about 2% available P2O5; soft, or colloidal, rock phosphate contains 3% available P2O5. Thus, a ton of these materials provides only 40 to 60 lb available P2O5/acre. Bone meal contains about 20 times more available P2O5 by weight, but is more expensive. Bone Char contains 16% available P2O5 and is less expensive than bone meal. With soils low in P, it can help crops to place proportionally more P fertilizer in the crop row than to broadcast it evenly. Maintain a pH of 6 to 7 with limestone to maximize P2O5 availability. Compost and manures tend to contain P2O5 as well as N or K2O. Repeated applications will raise P levels substantially and care must be taken to avoid building excessive P levels in the soil that could lead to contamination of ponds and lakes. Monitor P levels and adjust compost or manure applications accordingly.
Potassium
Sul-Po-Mag is the Potassium fertilizer of choice when Mg is also needed. Potassium sulfate is commonly used when no Mg is needed. Potassium becomes very slowly available from granite dust or greensand, which may be applied at 3 to 5 tons to the acre to build up K reserves. Wood ashes contain soluble K, but must be used with caution because they will raise the pH rather rapidly and can be caustic. The liming effect of 1 pound of ashes is roughly equal to 2/3 of a pound of limestone. No more than 1/2 ton of ashes per acre should probably be applied at once, and only then if called for by low pH, low K and sufficient Mg.
Magnesium
Magnesium is best applied as dolomitic lime, but when liming is not required, other Mg sources are Sul-Po-Mag or Epsom salts. Sul-Po-Mag is the better choice if potassium is also required, as it is less expensive than Epsom salts. However, Epsom salts can be applied as a foliar spray to alleviate Mg deficiency. Dissolve 1.5 lb per 10 gal water and spray at weekly intervals.
Micro-nutrients
Micro-nutrients are generally sufficiently supplied to plants by regular additions of organic matter to the soil. Wood ash is another excellent source of micronutrients. Some seaweed extracts may also supply trace minerals. In soils low in boron (B), remedial applications are widely recommended for crops that readily suffer from B deficiency. In this case, 1 to 2 lb/acre of B is applied to the soil with other fertilizers. It is difficult to apply such a small amount uniformly, but boron can be ordered as part of a fertilizer blend. Most boron products are soluble and can sprayed evenly over the soil. Several forms of B are listed by the Organic Materials Review Institute (OMRI), including Solubor, Fertibor and Biomin Boron. It is advisable to monitor B levels with soil tests and tissue tests (for perennial fruits). Excess levels of B are toxic to plants, and some crops are quite sensitive to boron.
Rock Powders
Rock powders can be used, along with organic matter, to build up and balance soil reserves of plant nutrients. However, these are not very soluble nutrient sources, and are not effective for treating short-term nutrient deficiencies. Using some soluble fertilizers may be advisable while building soil reserves of plant nutrients with rock powders and organic matter.
Limestone is a widely used rock powder. It raises the soil pH and provides calcium (Ca) and varying amounts of magnesium (Mg). When Mg tests below about 100 lb/acre, high-Mg limestone, or dolomite, should be used for liming. If Mg is above about 150 lb/acre, use calcite, or low-Mg lime. Choose your fertilizer materials considering the desired 20:4:1 base saturation ratio of Ca:Mg:K in the soil, but remember, this goal is only a ballpark figure and is definitely secondary to establishing the proper pH of 6 to 7 for most crops and supplying nutrients shown to be deficient by a soil test.