Twospotted Spider Mites
Mites belong to the order Acari of the class Arachnida (spiders, daddy-long legs etc.). Mites are very small and in contrast to insects, they are wingless and the head, thorax and abdomen appear to be fused together, generally giving the body a compact oval to oblong shape. Sex in many species is determined by fertilization; males develop from unfertilized eggs, females from fertilized eggs. The newly hatched young, called larvae, have only three pairs of legs. The larval stage is followed by two nymphal stages (a protonymph and a deutonymph) and by the adult stage. The nymphal and adult stages have four pairs of legs. Many species of mites are plant feeders and cause damage to crops with their piercing-sucking mouth parts including twospotted spider mite.
TWOSPOTTED SPIDER MITE, Tetranychus urticue
Damage
This species is most destructive under greenhouse conditions, feeding on over 300 plant species. Twospotted spider mites feed within plant cells causing damage to spongy mesophyll, palisade parenchyma and chloroplasts which results in reduced chlorophyll and the ability of the plant to photosynthesize. Light infestations cause stippling of leaves, especially of mature ones. Dulling and yellowing of foliage, followed by blasting of buds and flowers may occur as a result of heavy mite infestation.
The webbing of infested plant parts is one of the most characteristic signs of heavy mite infestation. The mite is most prolific at high temperatures (above 80°F) and at low humidity (20-40%), especially during the summer. At this time its high speed of reproduction may lead to sudden and destructive population explosions.
Description and Life Cycle
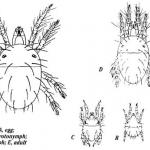
The adult female is oval, approximately 1/50-inch long, greenish to slightly orange, with two dark spots on either side of their body and four pairs of legs. The male is smaller than the female. Photo of adults and eggs by Leanne Pundt, UConn Extension
This mite prefers the lower surface of leaves, and bud and flower parts that offer protection. The mite spins thin strands of silk that develop into extensive sheets of webbing when the infestation is severe.
The globular or round shaped eggs are laid singly, up to 100 per female during her 3 to 4 week life span. When spider mite eggs are first laid, they are transparent and can be seen using a 10X handlens or magnifier. They turn straw colored near the time of hatching. The eggs hatch in as few as 3 days depending on temperature, and the newly hatched mites begin to feed immediately. Larvae are at first colorless and develop color as they feed. Their characteristic body spots are more distinct in the nymphal stage. After as few as 5 days, the mites pass through two nymphal stages (deutonymph and protonymph) and become adults. Spider mite adults are easily recognized by their two dark spots on either side of their body. Females begin laying eggs 1 to 3 days after emerging as adults, and mating is not required. The development from egg to adult may take as few as 7 days at 81°F and about 20 days at 64°F.
With decreasing day length in the fall, as well as falling temperatures and less available food, female spider mites enter diapause. Females entering diapause become an orange-red color within a few days of becoming an adult. They may overwinter in hoop houses and cooler greenhouses in a dormant stage. During this time, they do not eat, or lay eggs and are less susceptible to pesticides. They are also more difficult for predatory mites to find.
Management - Cultural
Continuous vigilance on the part of the grower may prevent a costly infestation. The introduction of infested plant material into greenhouses, as well as allowing vegetation to grow near the outer perimeter of the greenhouse, may give rise to population explosions, especially during the summer. Infestations usually start and develop most rapidly on plants near steam pipes or other objects which radiate heat. It is much easier for the grower to prevent a mite infestation than to eradicate it once it is established in the greenhouse. Pesticide resistance can be a common problem in spider mite control.
Cultural practices for managing twospotted spider mites includes carefully inspecting incoming plant material, removing weeds in and around greenhouses and disposing of old plant material including "pet plants" which may harbor populations. On some plants such as ivy geraniums, damage caused by thrips feeding and edema can look similar to damage caused by twospotted spider mites. Plants that are over-fertililized with nitrogen-based fertilizers promotes succulent new growth which is more susceptible to twospotted mites. Over-fertilized plants contain higher concentrations of proteins and amino acides that are essential food sources and may enhance development and reproduction of female twospotted mites. Twospotted spider mite populations may be higher in greenhouses that use only drip irrigation which keeps foliage dry. The use of occasional overhead irrigation will wash mites off plants.
Monitoring
Monitor plants weekly for signs of feeding injury. Look for mites on the undersides of mature leaves, especially along midveins. Gently tap leaves over a white piece of paper to dislodge mites to monitor more easily.
Routinely inspecting plants helps to keep track of the presence of the various life stages to avoid dealing with populations with all life stages present simultaneously. This in turn helps maximize the effectiveness (based on percent mortality) of any miticide applications. This may also reduce the number of applications needed, which is important because populations can easily develop resistance to miticides, making control or regulation very difficult. It is essential to rotate miticides with different modes of action in order to delay the onset of resistance.
Pesticide Use
Contact or translaminar miticides may be used to manage populations. For current pesticide information see the New England Greenhouse Floriculture Guide (under resources).
In general, contact miticides provide minimal residual activity once spray residues have dried. Thorough coverage of both the lower and upper leaf surfaces is critical when applying miticides with contact activity. Insecticidal soaps and horticultural oils are effective against twospotted mites; however, certain plants may be sensitive to both pest control materials. Consult the label to determine which plants to avoid spraying.
A number of miticides have translaminar activity, which mean that the material penetrates the leaf cuticle and the active ingredient resides within the leaf tissue including the spongy mesophyll and palisade parenchyma cells, resulting in a reservoir of active ingredient. This provides extended residual activity against twospotted spider mites even after spray residues have dried. Twospotted spider mites feeding on the leaves, even after spray residues have dissipated, may ingest a lethal dose of the active ingredient. This may lead to a decrease in the number of miticide applications, reducing worker exposure and minimizing the potential for spider mite populations developing resistance. Miticides with translaminar properties include abamectin (Avid®), etoxazole (TetraSan®), chlorfenapyr (Pylon®) and spiromesifen (Judo®). Read the label before making an application to determine the life stages (e.g., egg, larvae, nymph, and/or adult), which are most affected by the miticide.
Spray infested plants, then mark several plants and use a 10X hand-lens to detect the presence of live and dead twospotted mites. Most miticides are not effective against the egg stage, so repeat applications should be implemented every 5–7 days.
Mite growth regulators such as etoxazole (TetraSan®) must be applied before populations are extensive. This mite growth regulator may be tank mixed with a miticide that is active on adults.
Biological Control (from New England Greenhouse Floriculture Guide- see resources)
Commercially available predatory mites that may control TSM populations include Phytoseiulus persimilis, Galendromus occidentalis, Neoseiulus californicus, and Amblyseius (=Neoseiulus) fallacis. Each species requires and is adapted to different environmental conditions (e.g., temperature and relative humidity). For example, G. occidentalis, N. fallacis, and N. californicus tolerate warmer conditions (>85ºF or 29ºC) and a lower relative humidity (30–40%) than P. persimilis. Furthermore, these predatory mites generally persist when populations are low.
The effectiveness of predatory mites depends on population levels and alternate food sources. For example, P. persimilis only feeds on twospotted mites, while the other predatory mite species either feed on alternative hosts or prey, or on flower pollen. Some biological control suppliers offer a mixture of predatory mite species in a single container. Other commercially available predators of twospotted mites include the predatory midge, Feltiella acarisuga, and the ladybird beetle, Stethorus punctillum. Below are descriptions of the predators commercially available for control or regulations of twospotted mite populations:
Phytoseiulus persimilis: This is the most effective predatory mite for control or regulation of TSM populations. It has demonstrated efficacy in greenhouse-grown cucumbers and in mixed flower and foliage crops in Florida and Ohio, and cut roses in California and New Zealand. Phytoseiulus persimilis spreads among crops to locate twospotted mite colonies using odors emitted from infested plants. This is a specialist predatory mite that only uses twospotted mite as a food source, feeding on all life stages (eggs, larvae, nymphs, and adults). Phytoseiulus persimilis adults are bright red, pear-shaped with long legs, and are larger and more active than twospotted mites. Photo by Dan Gilrein, Cornell Adult females lay eggs that are approximately 2–3 times as large as twospotted mite female eggs. Both the adults and nymphs actively search plants for twospotted mite. Phytoseiulus persimilis is suitable for use in short-term crops such as bedding plants at release rates of 1–4 mites per ft2 per week. Treat both infested and adjacent areas. Initiate releases early when twospotted mite populations are low or when first detected. Two applications, one week apart, may be required. Make releases near infestations and concentrate releases near localized hot spots. The temperature must be around 68ºF (20ºC) with 75% relative humidity in order for this predatory mite to be effective. When temperatures are ≥86ºF (30ºC), P. persimilis cannot keep-up with the reproductive capacity of TSM. When relative humidity is <60%, predatory mite female eggs wither or fail to hatch. When scouting, look for shriveled, dried up spider mites plus the presence of predatory mites and their eggs. P. persimilis eggs are oval or "football shaped" and roughly twice the size of spider mite eggs. Eggs are laid close to a food source. When first laid, they are a transparent light pink shade and later turn darker. You may start to see the eggs about two weeks after releasing the beneficial (depending upon temperature). Furthermore, look for the predatory mites, which are pear-shaped and move quickly when disturbed. You can shake plants or plant parts over a white sheet of paper (8.5 x 11 inches or 22 x 28 cm) and observe the predatory mites. For localized hot spots P. persimilis is more effective when applied at a rate of 1–4 predatory mites per ft2 per week.
Pest control materials that have been demonstrated to be compatible with P. persimilis include spinosad (Conserve®), pymetrozine (Endeavor®) and clofentezine (Ovation®). However, bifenazate (Floramite®), spiromesifen (Judo®) and chlorfenapyr (Pylon®) may be harmful to P. persimilis. In addition, insecticidal soap is not compatible with P. persimilis, although there are no toxic affects three days after release.
Neoseiulus californicus: This selective predatory mite has a broader host or prey range than P. persimilis, and survives longer in the absence of prey, feeding on other plant-feeding mites and thrips. They may even feed on mold and nectar. Neoseiulus californicus is the appropriate choice under high temperatures and relative humidity, and when TSM populations are low. Neoseiulus californicus is most active at temperatures between 46–90ºF (8–32ºC) and relative humidity of 40–80%. However, when twospotted mite populations are low, populations of N. californicus tend to decline faster than P. persimilis. Neoseiulus californicus is compatible with the following pest control materials: bifenazate (Floramite®), chlorfenapyr (Pylon®), spiromesifen (Judo®) and spinosad (Conserve®).
Feltiella acarisuga: This small (1/16 inches or 2.0 mm) predatory midge feeds on twospotted mite. Adults live up to three days, and are active at night, resting during the day on leaf undersides. Females lay orange-to-red eggs among twospotted mite colonies that hatch in 3–5 days. The larvae are the only predaceous stage, feeding on all life stages (eggs, larvae, nymphs, and adults) of twospotted mite. After 5–7 days, larvae transition into a white, pupal stage, on the underside of leaves. Adults emerge from pupae, and although they do not feed, they can fly, allowing them to locate twospotted mite populations on hanging baskets or other locations within the greenhouse that are not accessible to predatory mites. Furthermore, F. acarisuga is more effective on greenhouse-grown tomatoes than P. persimilis because the sticky trichomes or hairs on the leaves impede the movement of P. persimilis; however, this does not seem to inhibit the ability of adult F. acarisuga to locate twospotted mite. The larvae can also forage on greenhouse-grown tomatoes whose leaves have glandular hairs. Optimum environmental conditions for survival include temperatures between 68–80ºF (20–26ºC), and 80% relative humidity. Extended periods of low relative humidity (below 60%) may reduce survival and reproduction. Feltiella acarisuga does not have a resting stage in winter, and is active year-round. This predatory midge is shipped as pupae and the adults emerge soon after arrival. When scouting, look for the nearly white pupal cases near the mid- rib on leaf undersides.
Miscellaneous natural enemies: Neoseiulus fallacis is a predatory mite that can survive low temperatures and prey availability, and is resistant to some pest control materials. It is useful for controlling or regulating twospotted mite populations in outdoor situations. Similar to P. persimilis, N. fallacis rapidly reduces extensive twospotted mite outbreaks.
Galendromus occidentalis, another predatory mite, tolerates a wide range of temperatures and relative humidity, and is well-adapted to outdoor conditions.
The small (1.0 to 1.5 mm long), black, predatory ladybird beetle, Stethorus punctillum, feeds on all life stages of twospotted mite. Adults can fly, allowing them to locate twospotted mite colonies that are not accessible to predatory mites.
The feasibility of implementing a biological control program for twospotted mite depends on the pest management tactics used for other insect pests including thrips, whiteflies, and aphids. If pest control materials with long-residual activity are applied, avoid releasing predatory mites for approximately four weeks since any residues may kill the predatory mites. However, any direct and indirect effects may vary depending on the pest control material. For example, spinosad (Conserve®) is compatible with most twospotted mite predators, but residues of abamectin (Avid®) may be toxic for up to 14 days after application. If a biological control program for thrips has been implemented, then it may be possible to use the predatory mites discussed above.
References and Resources:
Photos - New England Greenhouse Update photo gallery
Cloyd R. FloriBytes. Year V, Issue 2, June 2010.
https://u.osu.edu/joneslab/
Gentile A.G. and D.T. Scanlon. A Guide to Insects and Related Pests of Floricultural Crops in New England. University of Massachusetts Cooperative Extension System. Revised 1992. Out of print
New England Greenhouse Floriculture Guide: A Management Guide for Insects Diseases Weeds and Growth Regulators Twospotted Spider Mite Section by Raymond Cloyd, Kansas State University.
Tina Smith
Extension Greenhouse Crops and Floriculture Program
University of Massachusetts, Amherst
2013