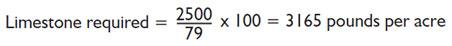Soil pH and Liming
What Does pH Measure?
Soil pH indicates the degree of soil acidity or alkalinity, and is reported using a scale ranging from zero to 14, with pH 7.0 being the neutral point. Soils with pH values below 7.0 are acid and above 7.0 are alkaline. Soil pH measures the hydrogen ion concentration in the soil solution, which is only a very small portion of the total hydrogen ions found in soil. The concentration in solution is referred to as the active acidity. The bulk of the hydrogen ion concentration is adsorbed to the soil's clay and organic matter particles and this is termed the soil's reserve acidity. The higher the clay and/or organic matter content a soil has, the greater is its capacity to hold hydrogen ions and therefore, the greater its reserve acidity. To place this in proper perspective, while it would only take 1/8 to 2 pounds of calcium carbonate (limestone) per acre to neutralize the hydrogen ions in soil solution (active acidity) it would take several tons of limestone per acre to neutralize the hydrogen ions held in reserve on the soil's exchange sites.
The Intensity and Capacity Factor of Soil Acidity
Soil pH can be thought of as an intensity factor that is analogous to air pressure in a tire. For example, we can have a bicycle tire and a tractor tire with the same air pressure just as we can have two different soils with the same pH. However, the air pressure does not tell us the amount of air that is in each tire with the same pressure, and the soil pH does not tell us the amount of hydrogen ions adsorbed to the exchange sites in the case of our two soils with the same pH. The amount of air that either tire would hold is different, as is obvious by the different sizes of the tires. This is termed the capacity factor, and is analogous to the soil's reserve acidity.
How Soils Become Acidic?
The major reason that soils are acid in the humid northeast is that leaching of the basic cations of calcium, magnesium, potassium and sodium leaves the exchange sites on the clay and humus particles open. Hydrogen ions replace the basic cations at the open exchange sites. Other natural processes which can lead to soil acidity include root respiration and the decomposition of organic matter by microorganisms. Human activity also causes soils to become acidic. Additions of ammonium containing fertilizers, sulfur containing ingredients in fertilizer and pesticides, and industrial activities that release sulfur and nitrogen oxides which find their way into soils through rainfall, all contribute to soil acidity. Removing grass clippings from turf areas carries away calcium and magnesium that would otherwise be recycled. Up to 40 pounds of calcium and 18 pounds of magnesium per acre per year can be removed in this manner. The degree by which human activity affects soil pH depends on the soil's ability to buffer against acid conditions.
Significance of Soil pH
Soil pH is one of the most commonly measured soil properties because it indicates processes that are taking place in soils, and their effect on plant growth. In strongly acid soils (soil pH less than 5.2) the availability of the essential elements such as calcium (Ca), magnesium (Mg), nitrogen (N) and phosphorous (P) as well as the micronutrients molybdenum (Mo) and boron (B) is reduced. In contrast, the elements of zinc (Zn), copper (Cu), iron (Fe), and manganese (Mn) are highly soluble in strongly acid soils and may even approach concentrations which are toxic to some plants. Aluminum (AI) toxicity to plants can also occur in strongly to very strongly acid soils, (pH 5.1) and below. Conversely, elements such as Zn, Fe, Cu and Mn are quite insoluble in slightly to moderately alkaline (pH 7.3 to 8.2) soils, which can lead to plant deficiencies for these elements. Phosphorus also forms insoluble compounds in strongly alkaline soils (pH >8.3) rendering it unavailable to plants. Heavy metals such as cadmium, lead, mercury and arsenic are highly mobile in strongly acid to very strongly acid soils, which increases their chance to move into groundwater. Maintaining soils in a slightly acid to near neutral condition through liming treatments reduces heavy metal mobility as they become adsorbed onto soil colloids. In addition, some pesticide reactions in soils are also governed by soil pH which affects their mobility. The adsorption of pesticides on some clays and humus colloids is enhanced as the soil pH increases due to an increase in the adsorptive capacity of these colloids.
Soil microorganisms are also affected by soil acidity. Fungi are adapted to a wide range of pH values while the bacteria and actinomycetes function best when soils are moderately acid to slightly alkaline. While there are many beneficial fungi, most turf pathogenic diseases are caused by fungi, so soils should be maintained in a slightly acid to neutral condition (pH 5.9 - 7.0). Conversely, fungi that cause take-all patch and pink snow mold disease are suppressed at a lower soil pH. The bacteria present in soils play a major role in decomposition of organic matter (including thatch), and nitrogen transformations such as nitrification and denitrification.
Soil acidity may also affect the composition of the turfgrass community, because of differences in species tolerance to soil pH. Bentgrasses and fine leaf fescues (Chewings, creeping red, hard and sheep fescues) are tolerant of acid soils (pH of 5.1 to 5.5) and often are provided a competitive advantage over other species when grown in soils with a low pH. Conversely, continued liming has been known to increase annual bluegrass populations in turf. Although it is difficult to generalize what the correct pH should be for culturing turfgrasses, maintaining a pH between 5.5 and 7.0 appears to be the most satisfactory range for most species. The final pH achieved should be dependent upon consideration of the factors above, such as nutrient availability, microorganism activity and species comprising the turfgrass community, particularly if the intent is to provide some species of turfgrass with a competitive advantage over others.
Buffer pH
Soil pH is usually determined in a slurry system using one part soil to one or two parts distilled water and then measuring pH with an electronic meter. However some soil test laboratories also report buffer pH along with soil pH values determined in distilled water. A buffer pH solution is used by these laboratories to determine the lime requirement (capacity factor) of the soil. It is a quick and easy method in which a buffer solution is added to the soil sample and the change in pH of the buffer (buffer solutions vary from pH 7.0 to 8.0) caused by the acids in the soil indicates the amount of lime required to neutralize the soil acidity. The more acid the soil, the greater the depression in buffer solution pH and therefore the greater the amount of lime required. Factors affecting the lime required to neutralize soil acidity include the soil's clay and organic matter content and type of clay present.
Limestone Recommendations
Limestone recommendations from a soil test laboratory are usually based upon incorporating the limestone to plow depth, approximately 7 inches. The obvious time to do this in turfgrass culture is at seedbed preparation during new construction. This may be the only time that limestone recommended by a laboratory can be applied in a single application. (See information on application to established turf on the next page.) Further, it should be stressed that the limestone be thoroughly mixed throughout the six to seven inches of soil. If the incorporation is shallower than this, limestone amounts should be adjusted according to Table 1.
| Depth of Incorporation in inches | Adjustment Factor |
|---|---|
| 3 | 0.4 |
| 4 | 0.6 |
| 5 | 0.7 |
| 7 | 1.0 |
Example: If the recommended limestone treatment is 2900 pounds per acre and incorporation is only to 4 inches deep then multiply the recommended rate by the adjustment factor 0.6:
Adjusted rate = 2900 x 0.6 = 1740 pounds limestone per acre incorporated to 4" depth.
A limestone application to established turf should not exceed 70 pounds per 1000 sq. ft. (1.5 tons per acre) in a single treatment. If the recommendation from the soil test laboratory is more than this, then the limestone should be applied in several treatments on a semiannual or annual basis until the quantity of limestone recommended by the laboratory is met.
Limestone applications to the turf on athletic fields and putting greens built with sand rootzones should not exceed 25 pounds per 1000 sq. ft. (1/2 ton per acre) in a single application. This approach ensures that the pH at the surface will not become alkaline. Agricultural limestone is slow to dissolve and it takes time for the calcium and magnesium to move down into the soil profile. Further, the calcium and magnesium are rapidly adsorbed to the clay and humus particles.
Limestone Quality and Recommended Adjustments
The value of limestone is based upon its acid neutralizing potential and its fineness guarantee. The acid neutralizing potential is expressed as the calcium carbonate equivalent that is printed on every limestone bag. The calcium carbonate equivalent is an expression of a limestone's purity and affects the actual amount of limestone required to neutralize soil acidity. Recommendations for limestone from a soil test laboratory are based on limestone with 100% pure calcium carbonate as the standard. However the agricultural limestone you purchase is never 100% pure because of natural impurities in the stone at the quarry. Therefore, to find the exact amount of limestone you will need to apply, the recommendation should be adjusted. This is done by dividing the laboratory recommendation for limestone by its calcium carbonate equivalent and then multiplying by 100.
- Calcium carbonate equivalent (CaCO3) on the bag = 85%
Laboratory recomended limestone treatment = 2500 pounds per acre - Calcium carbonate equivalent (CaCO3) on the bag = 79%
Laboratory recommended limestone treatment = 2500 pounds per acre
The finer a limestone is ground, the faster it will react with the soil to raise the soil pH. This is due to the greater surface area particles possess as they are ground finer and the fact that more limestone particles come in contact with more of the soil particles provided the limestone is uniformly mixed throughout the soil matrix. Some states have laws governing fineness guarantee.
Applying Limestone - How Much and How Frequently
The amount of limestone required to adjust the pH of the soil will depend on the soil type and the original soil pH. Soils with a high clay and organic matter content (greater reserve acidity) will require greater amounts of limestone to neutralize acidity than a sandy soil lower in clay content and organic matter (lower reserve acidity) given that each soil has the same pH to start with. For example, if we had a silt loam soil and a loamy sand in which each had a pH of 5.5 and we wished to raise the pH to 6.5 for each soil, it would require 1 to 1-3/4 tons of limestone per acre to adjust the soil acidity on the loamy sand. It would require 2-3/4 to 4 tons of limestone to raise the soil pH to 6.5 for the silt loam. The range in rates within each soil depends on their specific organic matter content.
The frequency at which limestone is applied depends on soil type and on soil drainage. A sandy soil needs to be limed more frequently because of its lower buffering capacity (holds less calcium and magnesium because of fewer exchange sites) than a soil higher in clay and organic matter. A soil that is poorly drained requires less frequent liming than a well drained soil because of the reduced leaching of basic cations under poorly drained conditions.
Managing soil pH on putting greens and athletic fields constructed with sand rootzones is a special case due to the sand rootzones' extremely poor buffering capacity. Using a topdressing material as part of routine maintenance whose pH value is near or above neutral (pH 7.0) may be all that is required to maintain the rootzone at pH 6.5 or above, particularly at the surface. On the other hand, routinely applying a topdressing material that is strongly acid (pH 5.1) may quickly change the pH in which the rootzone surface may become too acid thus requiring a light application of limestone. Care must be taken not to overlime these soils because they are more likely to be already deficient in micronutrients, and their availability may be further reduced from overliming.
Managing soil pH is an integral part of best management practices and requires knowledge of how soils become acid and the important role that pH has on the physical, chemical and biological properties of soils and subsequent turfgrass response and vigor. Giving close attention to this important soil property cannot be overemphasized.
--
Revised: 05/2011