A monthly e-newsletter from UMass Extension for landscapers, arborists, and other Green Industry professionals, including monthly tips for home gardeners.
To read individual sections of the message, click on the section headings below to expand the content.
To print this issue, either press CTRL/CMD + P or right click on the page and choose Print from the pop-up menu.
UMass Extension's Green School
Move your horticultural career or business forward!
UMass Extension’s Green School starts October 25th! This comprehensive 60+ hour certificate short course offers fundamental horticultural training in a compact time frame.
Why Choose Green School?
- A robust alternative or a stepping stone to a more involved degree program.
- Convenient yet rigorous remote learning from the comfort of your home or office.
- In-depth, research-based training for skills that are in high demand, and are applicable to active and engaging outdoor work.
- Three educational track options to fit your personal goals.
- Structured interaction with University educators and researchers.
- Competitive fees and tuition assistance options.
- An established program with a 30+ year history and a certificate that carries weight in the industry.
Who Should Attend? Professional practitioners such as landscapers, lawn care providers, nursery operators, sports field managers, public and private grounds managers, arborists, professional gardeners, landscape and garden designers, and others in the green industries. Both experienced individuals as well as those aspiring to be will benefit from this course.
Where? Entirely virtual in 2022, taught by UMass Extension Specialists, UMass faculty, and distinguished guest instructors.
When? Tuesday, Wednesday and Thursday afternoons 1:00 to 4:30 pm from 10/25/2022 thru 12/15/2022.
How? The curriculum emphasizes a systems-based approach to plant and land care based on current research and is built on a framework of Best Management Practices (BMPs) and Integrated Pest Management (IPM).
Choose from three specialty tracks:
- Landscape Management
- Turf Management
- Arboriculture
For complete information, detailed schedules and registration options, go to https://ag.umass.edu/landscape/education/umass-extensions-green-school
Hot Topics
Localized Dry Spot
Unusually dry weather this spring and summer made life hard for lawns in our area. Unirrigated turf (especially Kentucky bluegrass) in many places responded to these conditions by going into dormancy. Even irrigated turf felt the drought stress as low humidity led to a high rate of evapotranspiration.
There are several reasons why grass turns brown. If causes such as improper irrigation, inadequate cultural practices, insect pests, and diseases can be ruled out, the problem might be localized dry spot.
Localized dry spot can occur in prolonged periods of warm, dry weather. Under these conditions, soil microbes will produce hydrophobic substances that coat soil particles and prevent water penetration. This condition is typically seen in the top 1-2 inches of the soil.
Sandy soils and coarse soils are most susceptible to localized dry spot. Soil compaction and excess thatch may contribute to the development of this problem. Fairy rings are sometimes accompanied by dry spots.
Management of localized dry spot
- Ensure that your irrigation system is functioning properly and that coverage is adequate.
- Aerate to relieve soil compaction and excess thatch.
- Apply a surfactant (wetting agent), which enables water to more easily penetrate the soil.
- Get a soil test. High salinity can also lead to localized dry spot.
Angela Madeiras, UMass Extension Plant Pathologist
An Update about Neonicotinoid Use in Massachusetts
Beginning July 1, 2022 systemic insecticide active ingredients known as neonicotinoids have become state restricted use for tree and shrub uses in Massachusetts. If an individual works in the commercial industry (landscapers, arborists, etc.), then a Commercial Certification License is needed. (Example: Category 36 Commercial Certification License, Shade Trees & Ornamentals.) Someone can use a state or federal restricted use pesticide if they have a Commercial Applicators License as long as they are working under the direct supervision of someone with a Commercial Certification. Unlicensed or uncertified individuals will no longer be able to apply neonicotinoids to manage insect pests of trees and shrubs in Massachusetts.
More information is available at: https://www.mass.gov/service-details/pesticide-newsupdates
Helpful Information from Taryn LaScola-Miner, Director, Crop and Pest Services Division of the MA Department of Agricultural Resources:
“As you know, products that contain neonicotinoids and have certain use patterns will have a classification change from General Use to Restricted Use on July 1, 2022. In order to help inform the manufacturers, dealers, sellers and applicators of which products will be changing from general use to restricted use, the Massachusetts Department of Agricultural Resources (MDAR) has created the list of neonicotinoid products that currently are and will become restricted use beginning July 1st. You may find the list at the link below. Please note that this list is subject to change.
Additionally, MDAR is anticipating that there will be more of a need for companies to follow the Direct Supervision regulations with this change. Therefore, MDAR has updated its Direct Supervision Frequently Asked Questions document as well.
Although an email will be sent to all licensed applicators within the next few weeks as a final reminder of the change, please pass this information along to your members and customers as an effort to make this transition as smooth as possible. If you have any questions, please let me know. Thank you!”
List of Neonicotinoid Products: https://www.mass.gov/doc/list-of-neonicotinoid-pesticides/download
Direct Supervision Frequently Asked Questions: https://www.mass.gov/doc/direct-supervision-frequently-asked-questions-faq/download
Trouble Maker of the Month
Asian Longhorned Tick: Haemaphysalis longicornis
Species Overview
The Asian longhorned tick is an exotic, hard tick found in the United States in 2017. Genetic analysis shows it has likely been introduced into the country on three separate occasions. Since its initial discovery in 2017, it has undergone a rapid range expansion and has been found in at least 17 states, including New York, Connecticut, and Rhode Island. Make no mistake: If it isn’t in Massachusetts already, this tick is on our doorstep.
In its native range, two populations of Asian longhorned tick exist:
- A type that reproduces bisexually through mating.
- A type consisting of only females that reproduces parthenogenetically (without mating).
In older literature, the bisexual type was referred to as Haemaphysalis bispinosa. However, current data show that both are the same species. In the United States, only the parthenogenetic type has been found. Each female can lay up to 2,740 eggs.
The Asian longhorned tick has diverse tastes, feeding on many different animals. It prefers mid to large mammals, like cattle, horses, sheep, and goats, but will also feed on birds, rodents, cats, dogs, humans, and other animals... although it so far appears less attracted to humans. Both its reproductive methods and diverse tastes have allowed it to rapidly expand geographically. It can be moved around on a variety of animals and readily find food in new locations. And, it just takes a single tick to establish a population in a new location.
Asian longhorned tick is tolerant of dry conditions and can be found in locations ranging from forests to meadows. This species has been found in areas that you might not normally associate with ticks, such as railroads tracks, public parks, and backyard lawns.
Disease transmission
In its native range, the Asian longhorned tick has been associated with 30 human and animal pathogens, including some that are very serious. Before jumping to conclusions, additional research is necessary to understand the impact this tick has on human and animal health. Asian longhorned tick has not been shown to transmit Lyme disease. However, it has been found carrying the rare Bourbon virus in nature. It has also been shown to transmit the rare Heartland virus under controlled laboratory settings. Asian longhorned tick is strongly correlated with the Theileria orientalis Ikeda genotype in cattle. Dogs may be at risk for contracting Babesiosis caused by Babesia gibsoni.
Identification
The Asian longhorned tick is a small reddish, brown tick without patterning on its back, and is often found in very large numbers with overlapping life stages. This characteristic is somewhat unique among ticks encountered by people.
Identification is challenging. The Asian longhorned tick can look very much like the native rabbit tick (Haemaphysalis leporispalustris), but there are some distinct physical morphologies that separate it . However, these must be viewed using a microscope and require mouthparts to be intact. Accurate identification is best performed through DNA analysis.
If you come across any ticks fitting the description (color, prolific numbers), reports can be submitted to the New England Center of Excellence in Vector-Borne Diseases (NEWVEC) at https://www.newvec.org. Even if identification is uncertain, any information about potential sightings is helpful. Understanding where and when Asian longhorned tick comes to Massachusetts is important.
Management and Protection
When traveling to states where Asian longhorned tick is present (e.g. CT, RI, NY), be sure to check yourself and any animals to avoid bringing the tick back home. Farm animals, like horses, sheep, and cattle, may be particularly at risk because they fit the profile of “preferred hosts.”
If you protect against deer ticks, you will protect against Asian longhorned tick:
- Wear EPA-registered repellents. Look for these active ingredients in repellents:
- DEET
- Picaridin
- IR3535
- Oil of Lemon Eucalyptus·
- p-Menthane-3,8-diol
- 2-Undecanon
- Treat clothing and fabrics with permethrin to kill any ticks that come into contact with those items.
- Protect animals using flea and tick protection recommended by your veterinarian.
- A note about new oral medications (e.g. Nexgard, Braveto): Unlike topical treatments and collars, ticks need to initiate feeding to be affected by these. It is possible ticks could be removed from the animal before they are exposed to the chemical. If solely relying on oral medications, owners will need to be extra vigilant about checking for ticks to avoid bringing them to new locations where they can establish new populations.
- Be vigilant about doing regular tick checks. Remember, it only takes one tick to establish a population in a new area.
*Read and follow all instructions and safety precautions on labels.
Additional sources of information on the Asian longhorned tick:
- Centers for Disease Control (CDC) - https://www.cdc.gov/ticks/longhorned-tick/index.html
- United States Department of Agriculture (USDA) - https://www.aphis.usda.gov/aphis/maps/animal-health/asian-longhorned-tick
Blake Dinius, Entomologist, Plymouth County
Q&A
Q. I was so busy in May and June that I never got around to pruning my lilacs, azaleas and fragrant viburnums. Is it ok to prune them now? Will I do them any harm?
A. Pruning woody ornamentals now will not cause harm if you follow good pruning techniques (make a clean cut just above a leaf or branch union; leave branch collar intact; don’t remove more than 1/3rd of the overall foliage mass; favor out-facing shoots, etc.). In fact, corrective pruning to remove dead or diseased wood, rubbing or crossing branches can be done at any time. The drawback to pruning at this time of the year is that early flowering trees and shrubs, like those you’ve mentioned, and others like Weigela, flowering quince (Chaenomeles), Spiraea, and hybrid witch-hazels (Hamamelis x intermedia), have formed their flower buds for next spring already, so pruning now will mean the loss of some of those flowers. Late summer pruning, particularly as we get into September, sometimes stimulates a burst of growth which doesn’t properly harden off before winter sets in, so prune in August or hold off until the dormant season.
Q. I am seeing notches along the edges of leaves, as well as holes on many perennials and annuals but can’t find the culprit – I’ve looked under the foliage, on stems and at the soil line but can’t seem to find any caterpillars or Japanese beetles – help!
A. There are two likely candidates causing the damage during the evening hours. Both the Oriental and the Asiatic garden beetle are active at night (the Asiatic garden beetle is hidden by day), so the damage they cause often seems mysterious. Fortunately, the damage to the upper portions of plants is aesthetic and not life-threatening.
The Asiatic garden beetle (Maladera castanea) is a small, cinnamon-colored beetle often seen around outside lights and on screens at night. They are usually about 3/8” long. Using a flashlight, you can handpick them from plants at night and drop them into a dish of soapy water. They are usually not damaging enough to warrant any kind of chemical control. Females lay eggs in the soil; the larvae overwinter in the soil and feed on the roots of vegetation in spring and early summer. You can learn more about this beetle here.
The Oriental garden beetle (Anomala orientalis) is active during the day as well as at night. It looks like a dusky, faded Japanese beetle - tan in color often with stripes and darker markings which can be variable. The Oriental beetle emerges a week or two before the Japanese beetle and feeds on a similar range of plants, often found on the flowers. The visible damage from this beetle is usually light but, like the Japanese beetle, their grubs can cause significant damage to the roots of turf. Learn more here.
European chafers (Rhizotrogus majalis), a tan colored beetle which is a bit larger than a Japanese beetle, are also a nocturnal flyer and might be seen congregating around trees when you’re scouting for the Asiatic garden beetle. They do not generally feed on foliage and are most damaging to the roots of turf.
Q. I’d like to hold over some of the tender plants from my garden and containers – when is the right time to take cuttings and how should I do it?
A. August and early September is a great time to take cuttings of the plants you’d like to keep indoors for next year. It’s a great way to multiply your numbers of these plants, and to hold them over in a smaller, more manageable size.
Plants that lend themselves to this are those that are true perennials – not those with an annual life cycle (flower, set seed and perish in a single growing season). Annuals are best started from seed each year. Tender perennial tropical and subtropical plants that are simply not hardy enough for us to grow outdoors in our climate, and those that thrive in lower light conditions and low humidity, make good candidates for holding over in your home. Coleus (Solenostemon), Tradescantia, Fuchsia, tender geranium (Pelargonium), Salvia, and Plectranthus all root readily and can be held through the winter if you have the right indoor growing conditions. Here are a few tips on how to how to take cuttings:
- Have on hand: clean, sharp pruners, perlite and or vermiculite, rooting hormone, small pots or seed flats, and a plastic bag or film to make a small tent to maintain a humid environment.
- Harvest cuttings in the morning when plants are fully turgid (hydrated). Select non-flowering stems if possible and use only healthy, pest free plants.
- Using clean, sharp pruners, take several 4-6” long cuttings, making a cut just above a leaf axil.
- Process the cuttings right away, if possible. If you must delay, hold them in a plastic bag in the refrigerator.
- When ready to stick the cuttings, fill pots or seed flat with perlite or vermiculite and moisten.
- Trim the cutting to 3-4” or three sets of leaves. Remove lower set of leaves and make a clean cut just below where the leaves have been removed.
- Pinch out the growing tip and reduce leaf size by 1/3 if leaves are large.
- Dip the base of each stem in the rooting hormone.
- Make a hole for the cutting in the perlite using a pencil or similar implement that is slightly larger than the stem diameter.
- Gently place the cutting on the hole, burying to just below the remaining first set of leaves.
- Firm the perlite or vermiculite gently and water to ensure good stem contact.
- Enclose the pot and cuttings in a plastic film or bag to keep humidity high. Vent the enclosure twice a day to allow for fresh air circulation.
- Keep in a bright but shaded spot. Water as needed to keep the perlite moist.
- After 3-4 weeks, test to see if the plants have rooted by gently tugging to see if you feel any resistance from newly formed roots. Many of these easily propagated plants should root in 3-5 weeks and will be ready to pot up into potting soil. Hold on a sunny windowsill or under lights indoors over the winter.
Joann Vieira, Director of Horticulture, Trustees
Garden Clippings Tips of the Month
August is the month to . . . .
-
Water efficiently. With much of Massachusetts experiencing some level of drought or dry conditions, it is important to make sure irrigation is applied carefully and any local water restrictions are followed. Applying water to the base of plants helps ensure that water reaches the root zone where it can be used by plants and is not lost to evaporation. Irrigating early morning or later in the evening also reduces evaporation. Drip irrigation and soaker hoses can be used to apply irrigation more directly. Infrequent, deep irrigation is most beneficial.
-
Reapply mulch as needed. Mulch any bare spots to help reduce weeds, limit evapotransipiration, help regulate plant root temperature, and add organic mater to the soil.
-
Deadhead. Removing spent flower blooms from perennials and annuals can encourage rebloom and helps keep plants tidy. Perennials that can rebloom after deadheading include Echinacea purpurea, Monarda didyma, and Lavandula spp.
-
Keep weeding. Be sure to remove weeds before they go to seed. Don’t add weeds with seed heads to your compost. The pile may not get hot enough to kill the weed seeds.
-
Plant cool season crops. This includes broccoli, cabbage, and cauliflower starts. Sow seeds of beets, carrots, kale, or spinach.
-
Plan ahead for fall planting. Now is the time to place bulb orders for spring blooming bulbs for best selection.
-
Assess your landscape and plan for next year. Are there bare spots that need pops of color? Rudbeckia spp., Perovskia atriplicifolia, Coreopsis verticillata, Achillea spp. all continue to bloom through the heat of August. Clethera alnifolia has fragrant flowers and is a pollinator favorite!
-
Divide spring and summer blooming perennials. Late August and September is the time to divide perennials such as Paeonia spp. and Iris siberica. Be sure to allow 4-6 weeks for root establishment before freezing temperatures.
-
Assess annuals. Remove any that are no longer thriving as they won’t have time to recover before the end of the season.
-
Stop fertilizing roses. It is important to stop fertilizing roses to ensure growth stops before the first frost to reduce winter kill.
-
Stop pruning evergreens. Similar to roses, you don’t want to encourage new growth that will be sensitive to frost and won’t harden off before winter.
Mandy Bayer, Extension Assistant Professor of Landscape Horticulture
Sunflower Pollen and Bee Health: Research from the Adler Lab at UMass Amherst
In the February 2022 issue of Veg Notes, we provided an update on bee health. We described how native bee populations are declining due to habitat loss, disease, pesticides, and climate change. We also pointed out that the most effective way to help bees is to establish habitat; that is, maintain flowering plants for bees to eat and leave undisturbed areas for them to nest.
But which flowers are best to plant? We know that diversity is important; as a rule of thumb, that means floral mixes should include at least three plants that bloom each season. However, there is still much that we don’t know about how different flowers affect bee health. Pollen and nectar vary widely among plant species – in terms of nutritional composition, chemistry, and morphology – and this variation affects bees and bee pathogens in complex ways. The impact of floral diet on bee health is an active area of research globally and here in Massachusetts.
In this article, we will describe some novel research on bee habitat that is happening here at UMass Amherst. Dr. Lynn Adler and her lab study how floral diets affect bee diseases. In 2015, they found that common eastern bumble bees (Bombus impatiens) fed sunflower pollen had lower levels of a ubiquitous gut parasite (Crithidia bombi). They’ve since conducted numerous experiments to learn how and why sunflower pollen impacts bumble bee disease. Their goal is to understand the value of sunflower and related plants as part of a diverse floral mix. (You can read more about this research in this fact sheet).
Methods
The Adler Lab uses the model system of the common eastern bumble bee and their ubiquitous gut parasite Crithidia to answer questions about how pollen chemistry and shape affect bee disease.
The common eastern bumble bee (Bombus impatiens) is native to many parts of Eastern North America. Unlike most wild bees, bumble bees live in social colonies. Each colony is founded in the spring by a queen bee. She searches for a ground cavity, such as an abandoned rodent burrow, in which to establish her nest. She then lays eggs and provisions them with pollen and nectar. The colony grows to several hundred individuals over the summer. Most of the bees are female workers. Towards the end of the summer, the queen starts producing males and reproductive females (daughter queens) that leave the nest and mate. In the winter, the colony dies, and only the newly mated queens overwinter underground. Unlike many native bees, the common eastern bumble bee is not in decline. In fact, populations have been increasing. However, it is an ideal organism to study because it is reared commercially for greenhouse pollination so can be purchased easily and maintained indoors. Colonies can also be reared in the lab from wild-caught bees. In addition, this bee is important because it is very abundant and likely transmits diseases to other bees.
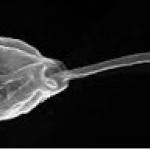 The pathogen Crithidia (Crithidia bombi) is a gut parasite of bumble bees. Crithidia is a protozoan pathogen that lives in bee intestines and is transmitted through feces in the colony or on flowers. Studies find that it is ubiquitous among bumble bees in Massachusetts. It reduces bumble bee longevity, learning, foraging and queen hibernation. It is useful to study because it is widespread and can be easily maintained in a lab.
The pathogen Crithidia (Crithidia bombi) is a gut parasite of bumble bees. Crithidia is a protozoan pathogen that lives in bee intestines and is transmitted through feces in the colony or on flowers. Studies find that it is ubiquitous among bumble bees in Massachusetts. It reduces bumble bee longevity, learning, foraging and queen hibernation. It is useful to study because it is widespread and can be easily maintained in a lab.
The common sunflower (Helianthus annuus) is native to North America and its yield is improved by bee pollination. Sunflowers are cultivated worldwide for their oil; in 2018, there were over 1.2 million acres planted in the United States. Sunflower pollen is relatively low in protein, so bees fed exclusively sunflower have smaller larvae and shortened lifespans. However, when bees eat 50% sunflower pollen mixed with pollen from other flowers, they live as long as bees fed non-sunflower pollen. Sunflower pollen is notable because it has a spiky exterior, like pollen from many plants in the same family (Asteraceae).
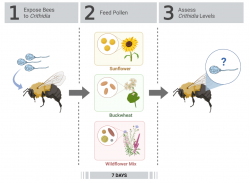 For all lab experiments, bumble bees were exposed to Crithidia, fed different pollen diets, and then dissected to assess Crithidia levels. Figure 1 shows the experimental method for Giacomini et al. 2018 (described below in “1. Main Finding”). Variations to this method are indicated for each result.
For all lab experiments, bumble bees were exposed to Crithidia, fed different pollen diets, and then dissected to assess Crithidia levels. Figure 1 shows the experimental method for Giacomini et al. 2018 (described below in “1. Main Finding”). Variations to this method are indicated for each result.
Results
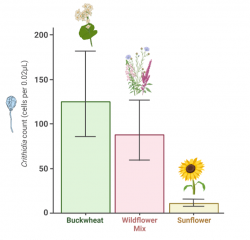 1. Main finding
1. Main finding
Sunflower pollen dramatically reduced Crithidia bombi in both wild and commercial Bombus impatiens. After exposing bees to Crithidia and feeding them different pollen diets (see Figure 1), Adler Lab researchers consistently found that bees fed sunflower pollen had dramatically lower levels of Crithidia than bees fed buckwheat or wildflower pollen (Figure 2). Because buckwheat pollen, like sunflower pollen, is low in protein, the researchers also concluded that sunflower’s low protein is not the reason it reduced Crithidia. Read the full paper here.
2. Do we see this effect outside the lab?
Yes! Bumble bees on farms with more sunflower had less Crithidia. In 2015, researchers gathered wild bumble bees from farms around the Pioneer Valley. They measured the area of sunflowers grown at each farm and assessed the bees’ Crithidia infection. They found that bees caught on farms with more sunflower had lower infection. Read the full paper here. In 2019, researchers placed commercial bumble bee colonies on Pioneer Valley farms with varying amounts of sunflower. At the end of the experiment, they assessed colony growth and Crithidia infection. They found that colonies placed on farms with more sunflowers had less Crithidia and reproduced more than colonies on farms with fewer sunflowers (Malfi et al., in prep).
3. How broad is this effect?
 Many different types of sunflower pollen reduced Crithidia in B. impatiens, including goldenrod, which is in the sunflower family but is not that closely related. Researchers exposed bees to Crithidia and then fed them nine varieties of commercial sunflower, four varieties of wild sunflower and two species of goldenrod, a distant sunflower relative. They found that all sunflower or goldenrod pollen reduced Crithidia 20-40-fold when compared to buckwheat pollen (and most reduced Crithidia compared to wildflower pollen) (Figure 3). This is important because it implies that sunflower’s medicinal qualities may extend to other members of the sunflower family, a diverse plant group found all over the world. Read the full paper here.
Many different types of sunflower pollen reduced Crithidia in B. impatiens, including goldenrod, which is in the sunflower family but is not that closely related. Researchers exposed bees to Crithidia and then fed them nine varieties of commercial sunflower, four varieties of wild sunflower and two species of goldenrod, a distant sunflower relative. They found that all sunflower or goldenrod pollen reduced Crithidia 20-40-fold when compared to buckwheat pollen (and most reduced Crithidia compared to wildflower pollen) (Figure 3). This is important because it implies that sunflower’s medicinal qualities may extend to other members of the sunflower family, a diverse plant group found all over the world. Read the full paper here.
Sunflower pollen reduced Crithidia in queens and workers, but not males. Researchers tested whether sunflower affects bee castes differently. They found that it reduced Crithidia in females (queens and workers) but not males. The effect on queens is important because daughter queens that emerge in the fall establish new colonies the following spring. Males had relatively low Crithidia levels regardless of diet, which may be because they consume very little pollen, and Crithidia is low when bees don’t eat pollen. Read the full paper here.
Sunflower pollen did not reduce Crithidia as dramatically in other bumble bee species. Researchers tested whether sunflower pollen reduced Crithidia infections in three other wild bumble bee species: Bombus griseocollis, Bombus bimaculatus, and Bombus vagans. They found that it slightly reduced Crithidia infection in B. bimaculatus and B. vagans and did not reduce infection in B. griseocollis. B. impatiens, B. bimaculatus and B. vagans are more closely related to each other than they are to B. griseocollis. Read the full paper here.
A 50% sunflower pollen diet reduced Crithidia infections. Dr. Adler’s collaborator Becky Irwin and her team at NC State University tested whether sunflower pollen still reduced Crithidia when combined with wildflower pollen. This is important because sunflower pollen is low in protein, and bees cannot eat exclusively sunflower pollen for long periods of time. The team fed individual bees either 100% sunflower pollen, 50% sunflower pollen, 25% sunflower pollen, or 100% wildflower pollen. They found that even a 50% sunflower diet reduced Crithidia infections. Read the full paper here.
4. Why does sunflower reduce Crithidia?
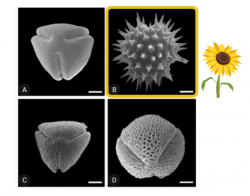 Sunflower may reduce Crithidia because of its spiky outer shell. Researchers in the lab isolated different chemical components of sunflower pollen (nine fatty acids and two defensive compounds) and fed them individually to bumble bees. They found that none of the compounds individually reduced Crithidia. Read the full paper here. Then they separated the spiky shell from the other pollen components and fed it to bees. They found that the hollow spiky shell alone reduced Crithidia as much as whole sunflower pollen, pointing to a physical mechanism. This may explain why pollen from other plants in the sunflower family (which also have spiky pollen) reduce Crithidia (Figueroa et al., in prep).
Sunflower may reduce Crithidia because of its spiky outer shell. Researchers in the lab isolated different chemical components of sunflower pollen (nine fatty acids and two defensive compounds) and fed them individually to bumble bees. They found that none of the compounds individually reduced Crithidia. Read the full paper here. Then they separated the spiky shell from the other pollen components and fed it to bees. They found that the hollow spiky shell alone reduced Crithidia as much as whole sunflower pollen, pointing to a physical mechanism. This may explain why pollen from other plants in the sunflower family (which also have spiky pollen) reduce Crithidia (Figueroa et al., in prep).
Take-away
- Sunflower (and related plants like goldenrod) may reduce a pervasive gut parasite in the common eastern bumble bee. This species is not in decline, but it is quite common, so may spread diseases to other species.
- However, sunflower is relatively low in protein, so is best for bees as part of a diverse wildflower planting.
- So…should you plant sunflowers to help bees? YES! But don’t forget to plant other flowers as well, to provide a diverse diet for your local pollinators. For more info on planting for bees, click here.
Next steps
- The lab is exploring how commercially grown cut flowers in the sunflower family (Asteraceae) affect bumble bee health. This research could help us understand the impact of cut flower production on bees, and whether cut flowers could be a way to improve habitat for bees in agricultural areas.
- The lab is also assessing the value of pollen from different native plants, which could be used to create pollinator habitat in conservation areas.
- They are also collaborating with ecologists and molecular biologists to understand how pollen affects Crithidia at a molecular level, how plants affect disease transmission, and what plants are visited most by bees, in order to model how plant communities affect bee health.
Hannah Whitehead, UMass Extension Vegetable Program
Upcoming Events
For more details and registration options for upcoming events, go to the UMass Extension Landscape, Nursery, and Urban Forestry Program Upcoming Events Page.
- 9/15/22 - End of early-bird rate for Green School registrations. For complete details and registration options, go to https://ag.umass.edu/landscape/education/umass-extensions-green-school
- 10/18/22 - Deadline for registering for Green School
Pesticide Exam Preparation and Recertification Courses
These workshops are currently being offered online. Contact Natalia Clifton at nclifton@umass.edu or go to https://www.umass.edu/pested for more info.
InsectXaminer!
Episodes so far featuring gypsy moth, lily leaf beetle, euonymus caterpillar, imported willow leaf beetle, and spotted lanternfly can be found at: https://ag.umass.edu/landscape/education-events/insectxaminer
TickTalk with TickReport Webinars
To view recordings of past webinars in this series, go to: https://ag.umass.edu/landscape/education-events/ticktalk-with-tickreport-webinars
Additional Resources
For detailed reports on growing conditions and pest activity – Check out the Landscape Message
For professional turf managers - Check out our Turf Management Updates
For commercial growers of greenhouse crops and flowers - Check out the New England Greenhouse Update website
For home gardeners and garden retailers - Check out our home lawn and garden resources.
Diagnostic Services
Landscape and Turf Problem Diagnostics - The UMass Plant Diagnostic Lab is accepting plant disease, insect pest and invasive plant/weed samples . By mail is preferred, but clients who would like to hand-deliver samples may do so by leaving them in the bin marked "Diagnostic Lab Samples" near the back door of French Hall. The lab serves commercial landscape contractors, turf managers, arborists, nurseries and other green industry professionals. It provides woody plant and turf disease analysis, woody plant and turf insect identification, turfgrass identification, weed identification, and offers a report of pest management strategies that are research based, economically sound and environmentally appropriate for the situation. Accurate diagnosis for a turf or landscape problem can often eliminate or reduce the need for pesticide use. See our website for instructions on sample submission and for a sample submission form at https://ag.umass.edu/services/plant-diagnostics-laboratory. Mail delivery services and staffing have been altered due to the pandemic, so please allow for some additional time for samples to arrive at the lab and undergo the diagnostic process.
Soil and Plant Nutrient Testing - The lab is accepting orders for Routine Soil Analysis (including optional Organic Matter, Soluble Salts, and Nitrate testing), Particle Size Analysis, Pre-Sidedress Nitrate (PSNT), and Soilless Media (no other types of soil analyses available at this time). Testing services are available to all. The lab provides test results and recommendations that lead to the wise and economical use of soils and soil amendments. For updates and order forms, visit the UMass Soil and Plant Nutrient Testing Laboratory web site.
Tick Testing - The UMass Center for Agriculture, Food, and the Environment provides a list of potential tick identification and testing options at: https://ag.umass.edu/resources/tick-testing-resources.
![H. longicornis [Photo courtesy of Cumbie, A. N., Whitlow, A. M., Arneson, A., Du, Z., & Eastwood, G. (2022)] H. longicornis [Photo courtesy of Cumbie, A. N., Whitlow, A. M., Arneson, A., Du, Z., & Eastwood, G. (2022)]](/sites/ag.umass.edu/files/styles/150x150/public/newsletters/images/haemaphysalis_longicornis_geastwood.jpg?itok=Rg0K4eio)