A monthly e-newsletter from UMass Extension for landscapers, arborists, and other Green Industry professionals, including monthly tips for home gardeners.
To read individual sections of the message, click on the section headings below to expand the content.
To print this issue, either press CTRL/CMD + P or right click on the page and choose Print from the pop-up menu.
Gift Ideas for Gardeners
 Mass Aggie Seminar Series
Mass Aggie Seminar Series
Give the gift of learning! This educational series highlights the agricultural expertise and innovation available from the University of Massachusetts Amherst’s Extension Fruit Team. Through this series of webinars and workshops, the Fruit Team provides a platform for small scale backyard gardeners and agricultural enthusiasts of all types to come together to learn the latest developments in growing fruit. Delve into the cutting-edge information shared in our seminars, curated to empower individuals with the tools and knowledge needed to navigate the ever-evolving landscape of agriculture.
Cost: $35-$60/ea. Topics include: Disease in the Orchard, Home Orchard Establishment, For the Love of Pollinators, Integrated Pest Management & Insect ID, and Orchard Pruning.
For more details and to register yourself or someone as a gift, go to https://ag.umass.edu/fruit/news-events/mass-aggie-seminars-2024
 The UMass Soil and Plant Nutrient Testing Lab is excited to announce our first ever Soil Test Gift Kit!
The UMass Soil and Plant Nutrient Testing Lab is excited to announce our first ever Soil Test Gift Kit!
Each gift kit includes directions for collecting and submitting a sample, a sample bag, and a sample envelope (not pre-paid) to return the sample once it is collected. The cost per kit is $27, which includes the cost of a routine soil test with fertility recommendations ($20), a soil organic matter test ($6), and postage and handling ($1). The gift kit will be mailed back to you, the gift giver, in a mailer envelope. Inside the mailer, each kit will be in an envelope suitable for gifting. We are not able to ship to a gift receiver, so please leave a week for us to mail the kit to you after we receive your order, plus time for you to get it to your lucky person.
This offer is only available through 12/31/23. To purchase your gift kit, use this Soil Test Gift Kit Order Form. Additional information on fulfillment is included on the form.
 2024 UMass Garden Calendar
2024 UMass Garden Calendar
UMass Extension works with the citizens of Massachusetts to help them make sound choices about growing, planting, and maintaining plants in our landscapes, including vegetables, backyard fruits, and ornamental plants. Our 2024 calendar continues UMass Extension’s tradition of providing gardeners with useful and practical information. Many people also love the daily tips and find the daily sunrise/sunset times highly useful!
Cost: $14.50/ea plus shipping; bulk rates apply to orders of 10 copies or more. For images in the calendar, details, and ordering info, go to http://ag.umass.edu/gardencalendar
USDA Unveils Updated Plant Hardiness Zone Map
The U.S. Department of Agriculture (USDA) released in November a new version of its Plant Hardiness Zone Map (PHZM), updating this valuable tool for gardeners and researchers for the first time since 2012. USDA’s Plant Hardiness Zone Map is the standard by which gardeners and growers can determine which plants are most likely to thrive at a location. The new map—jointly developed by USDA's Agricultural Research Service (ARS) and Oregon State University's (OSU) PRISM Climate Group—is more accurate and contains greater detail than prior versions.
In addition to the map updates, the Plant Hardiness Zone Map website was expanded in 2023 to include a “Tips for Growers” section, which provides information about USDA ARS research programs of interest to gardeners and others who grow and breed plants.
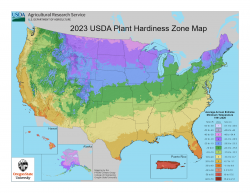 The 2023 map is based on 30-year averages of the lowest annual winter temperatures at specific locations, is divided into 10-degree Fahrenheit zones, and further divided into 5-degree Fahrenheit half-zones. Like the 2012 map, the 2023 web version offers a Geographic Information System (GIS)-based interactive format and is specifically designed to be user-friendly. Notably, the 2023 map delivers to users several new, significant features and advances. The 2023 map incorporates data from 13,412 weather stations compared to the 7,983 that were used for the 2012 map.
The 2023 map is based on 30-year averages of the lowest annual winter temperatures at specific locations, is divided into 10-degree Fahrenheit zones, and further divided into 5-degree Fahrenheit half-zones. Like the 2012 map, the 2023 web version offers a Geographic Information System (GIS)-based interactive format and is specifically designed to be user-friendly. Notably, the 2023 map delivers to users several new, significant features and advances. The 2023 map incorporates data from 13,412 weather stations compared to the 7,983 that were used for the 2012 map.
Plant hardiness zone designations represent what’s known as the “average annual extreme minimum temperature” at a given location during a particular time period (30 years, in this instance). Put another way, the designations do not reflect the coldest it has ever been or ever will be at a specific location, but simply the average lowest winter temperature for the location over a specified time. Low temperature during the winter is a crucial factor in the survival of plants at specific locations.
As with the 2012 map, the new version has 13 zones across the United States and its territories. Each zone is broken into half zones, designated as “A” and “B.” For example, zone 7 is divided into 7a and 7b half zones. When compared to the 2012 map, the 2023 version reveals that about half of the country shifted to the next warmer half zone, and the other half of the country remained in the same half zone. That shift to the next warmer half zone means those areas warmed somewhere in the range of 0-5 degrees F; however, some locations experienced warming in the range of 0-5 degrees F without moving to another half zone.
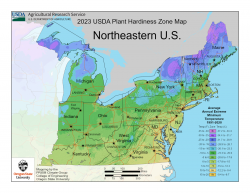 These national differences in zonal boundaries are mostly a result of incorporating temperature data from a more recent time period. The 2023 map includes data measured at weather stations from 1991 to 2020. Notably, the 2023 map for Alaska is “warmer” than the 2012 version. That’s mainly because the new map uses more data representing the state’s mountain regions where, during winter, warm air overlies cold air that settles into low-elevation valleys, creating warmer temperatures.
These national differences in zonal boundaries are mostly a result of incorporating temperature data from a more recent time period. The 2023 map includes data measured at weather stations from 1991 to 2020. Notably, the 2023 map for Alaska is “warmer” than the 2012 version. That’s mainly because the new map uses more data representing the state’s mountain regions where, during winter, warm air overlies cold air that settles into low-elevation valleys, creating warmer temperatures.
The annual extreme minimum temperature represents the coldest night of the year, which can be highly variable from year to year, depending on local weather patterns. Some changes in zonal boundaries are also the result of using increasingly sophisticated mapping methods and the inclusion of data from more weather stations.
Temperature updates to plant hardiness zones are not necessarily reflective of global climate change because of the highly variable nature of the extreme minimum temperature of the year, as well as the use of increasingly sophisticated mapping methods and the inclusion of data from more weather stations. Consequently, map developers involved in the project cautioned against attributing temperature updates made to some zones as reliable and accurate indicators of global climate change (which is usually based on trends in overall average temperatures recorded over long time periods).
Although a paper version of the 2023 map will not be available for purchase from the government, anyone may download the new map free of charge and print copies as needed.
This information was taken from a USDA news release.
Alternatives to Peat
One hot topic in the horticultural world these days is the effort to pursue viable alternatives to peat moss for use in starting seeds, growing container plants, and improving garden soil. While peat has certainly been seen as the gold standard, other contenders--old and new—are drawing the attention of horticultural researchers, commercial growers, and home gardeners. Using peat alternatives may require adjustments in terms of cultural practices, such as watering and fertilizing, but are worth trying for their benefits to plant growth.2
Peat: Consistent, Reliable
What is peat and why is it so widely used in the first place? Peat is a spongy material, consisting of partially decayed organic materials, primarily plants, that slowly accumulate under acidic, anaerobic conditions in certain types of wetlands, e.g. bogs. Its properties make peat an ideal growing medium. Peat contains numerous air spaces that enable oxygen to reach plant roots and that permit drainage of excess moisture. At the same time, peat can absorb water up to 20 times its own dry weight and hold that water for plant uptake. It can hold nutrients from fertilizers and release them as needed to plants. Peat has an acidic pH and needs to be amended with a material such as limestone to raise the pH for growing most plants. Peat is sterile—no weed or pathogen concerns—which is critical for seed starting.1,6 In addition, peat is lightweight and easy to purchase as compressed bales which expand for producing quantities of potting mix or amending soil over large garden areas.
Peat Alternatives: Experimental, Promising
The range of commercially available alternatives has been greater across Europe and the United Kingdom due to legislation restricting the use of peat in horticultural media, but reduced-peat and peat-free materials are becoming more widely offered in the United States.8
Some of these include:
Coir: Coir is a fibrous material from the hulls of coconuts, generated as a byproduct during coconut processing. Like peat, it holds water well and still allows for air movement around plant roots. It has a slightly acidic to neutral pH, is able to retain added nutrients and supply them as needed, and “recipes” abound online for coir-based planting mixes. Quality of coir products may be somewhat inconsistent and, in the past, high salt content from low-grade manufacturing was an issue.3,11 However, more coir-containing products from reputable horticultural brands are entering the market, from lightweight, compressed blocks of pure coir to mixes consisting of coir plus some permutation of other ingredients, e.g. peat, perlite, and/or nutrients.
Wood fiber: Wood-based mixes are another alternative to straight peat growing media. Researchers at North Carolina State University have found that fibers from trees (softwoods such as loblolly pine, spruce, fir, and larch) blended with peat make a successful mix: easier to moisten and keep moist than peat alone, while still promoting drainage and aeration around roots. The surface of containers filled with a wood fiber-peat mix dries out more readily than those without fiber, a factor both good (fewer surface pests) and bad (easier to overwater containers mistakenly thought to be dry throughout). And wood fiber mixes tend to have a higher pH than peat-perlite mixes, which can require adjustments in fertilizer application.5 Wood fibers are commercially available pre-mixed with peat and other amendments as well as unmixed for creating custom mixes.
Compost and bark: Spreading composted organic materials (plant residue, grass clippings, leaves, kitchen scraps) in garden beds is nothing new for most gardeners, though substituting them for a proportion of peat in container mixes may be less commonly practiced. Compost provides excellent moisture retention and is a natural source of plant nutrients. Commercially prepared compost-based mixes are sold for seed starting but homemade compost is generally not recommended for seed starting because of its non-sterile nature. Potential problems can arise in containers if the compost is inadequately decomposed (causing a lack of nitrogen availability, or the release of phytotoxic compounds), but using fully composted materials or commercially produced compost can alleviate issues.7,10 Bark, similarly, is best used when it has been properly composted to improve drainage in peat, compost, or other substrate blends. And when finely ground, pine bark has similar properties to peat- acidic, moisture-retentive, and long-lived.4,9,10
Other peat alternatives: Mixes containing materials such as paper fibers and dairy waste fibers are commercially available and, as research continues, more products will undoubtedly find their way into the market.
References
- ND. A detailed look at peat moss. Floriculture.
- ND. Alternatives to peat.
- 2007. Coir offers pros, cons. Nursery Management.
- Cantin, Brian. 2016. The benefits of bark. GrowerTalks.
- Jackson, Brian. 2016. Wood works as a peat option. GrowerTalks.
- Kitir, Nurgül & Yildirim, Ertan. 2018. Peat Use in Horticulture. 10.5772/intechopen.79171.
- Porter, John. 2019. Compost in seed starting mix: Recipe for success…or failure?
- Roach, Margaret. 2022. Why Gardeners Should Stop Using Peat, and What to Use Instead.
- Texas A&M. ND. Ornamental production: Growing media.
- University of Florida IFAS Extension. ND. Homemade potting mix.
- Washington State University. 2018. Coconut Coir vs Peat Moss.
Jennifer Kujawski, Horticulturist
Trouble Maker of the Month
Niveoporofomes
 Niveoporofomes is a lesser-known but common root and butt rot pathogen of oaks in the Northeast (Luley 2022). While N. spraguei primarily occurs on oak, it has also been documented on cherry, chestnut, maple and beech. Infections by Niveoporofomes result in a brown cubical rot of the heartwood in the roots and lower trunk. The decay column can occasionally progress upwards in the trunk to significant heights. Because the decay is mostly restricted to the heartwood, infected trees often appear healthy and exhibit no symptoms of the disease. At times, excessive basal tapering (or flaring) may be the only external symptom present. The pattern of decay (brown rot) preferentially targets cellulose, resulting in major reductions in wood bending strength (Schwarze et al. 2000). This can seriously compromise the tree's structural stability when the decay is offset from the center or encompasses a large percentage of cross-sectional area in the roots and lower trunk. Infected trees may be susceptible to uprooting or stem breakage under loading from strong winds. Overall, N. spraguei is poorly studied and much remains unknown about the rate the decay and the incidence of advanced infections in urban trees. Like many other wood decay fungi, Niveoporofomes can persist as a saprophyte within infected stumps and roots.
Niveoporofomes is a lesser-known but common root and butt rot pathogen of oaks in the Northeast (Luley 2022). While N. spraguei primarily occurs on oak, it has also been documented on cherry, chestnut, maple and beech. Infections by Niveoporofomes result in a brown cubical rot of the heartwood in the roots and lower trunk. The decay column can occasionally progress upwards in the trunk to significant heights. Because the decay is mostly restricted to the heartwood, infected trees often appear healthy and exhibit no symptoms of the disease. At times, excessive basal tapering (or flaring) may be the only external symptom present. The pattern of decay (brown rot) preferentially targets cellulose, resulting in major reductions in wood bending strength (Schwarze et al. 2000). This can seriously compromise the tree's structural stability when the decay is offset from the center or encompasses a large percentage of cross-sectional area in the roots and lower trunk. Infected trees may be susceptible to uprooting or stem breakage under loading from strong winds. Overall, N. spraguei is poorly studied and much remains unknown about the rate the decay and the incidence of advanced infections in urban trees. Like many other wood decay fungi, Niveoporofomes can persist as a saprophyte within infected stumps and roots.
 Niveoporofomes produces fleshy (but durable) annual conks consisting of one to several overlapping caps that are typically produced directly from the infected trunk. However, they can also occur on lateral roots close to the flare. The cap may be white to gray with a reddish-brown margin when fresh. In some cases, the entire conk is reddish-brown. Young conks will often exude water droplets from both the upper and lower surfaces. In Massachusetts, fruiting bodies typically first appear from late July into August. Over time, the water droplets dry and colors fade, giving the conks a bleached gray appearance. The pore layer is off-white and typically becomes greenish when bruised and handled. The context is zonate and has a marbled appearance that persists even when the conks are older. The fruiting bodies may be highly distorted in appearance if the site is exposed and dry. The presence of conks is not a reliable indicator of infection as trees may harbor advanced infections with no conks.
Niveoporofomes produces fleshy (but durable) annual conks consisting of one to several overlapping caps that are typically produced directly from the infected trunk. However, they can also occur on lateral roots close to the flare. The cap may be white to gray with a reddish-brown margin when fresh. In some cases, the entire conk is reddish-brown. Young conks will often exude water droplets from both the upper and lower surfaces. In Massachusetts, fruiting bodies typically first appear from late July into August. Over time, the water droplets dry and colors fade, giving the conks a bleached gray appearance. The pore layer is off-white and typically becomes greenish when bruised and handled. The context is zonate and has a marbled appearance that persists even when the conks are older. The fruiting bodies may be highly distorted in appearance if the site is exposed and dry. The presence of conks is not a reliable indicator of infection as trees may harbor advanced infections with no conks.
Management
As with any tree infected by a root and butt rot pathogen, maintaining high tree vigor is important. If annual increment growth can keep pace with the rate of decay in the heartwood, this may reduce the risk of failure. Maintain a large ring of mulch or wood chips around the tree and manage insects pests and pathogens that can weaken the tree. Improving soil quality may also increase vigor. Pruning to reduce canopy sway can also be performed. Regularly scout oaks for the presence of fruiting bodies and document their occurrence on and around the lower trunk. Conks may appear annually for many years around the base of infected oaks. Minimally invasive decay detection techniques, such as resistance drilling and sonic tomography, are often required to understand the extent of the decay. Tomography scanning of infected trees in Massachusetts has revealed dramatic differences in decay severity when conks are present. In some cases, trees with only a single conk have significant lower trunk rot, while at other times, trees with multiple conks at the base have no detectable decay in the lower trunk. This serves as an important reminder that the presence of conks does not always indicate serious decay is present in the lower trunk.
Additional photos of root and Butt rot caused by Niveoporofomes spraguei can be found here.
References
Luley, CJ. 2022. Niveoporofomes spraguei. In: Wood Decay Fungi Common to the Northeast & Central United States, 2nd Edition. Urban Forest Diagnostics LLC, Naples, NY. Pp. 72–73.
Schwarze FWMR, Engels J, and Mattheck C. 2000. Fungal Strategies of Wood Decay in Trees. Springer, Berlin, Germany.
Nicholas J. Brazee, UMass Extension Plant Pathologist
Q&A
Q: These larvae were found in containerized yellowwood (Cladrastis kentukea) that have spent one season in a nursery setting. The plants have poor rooting with many white grubs in the lower container media. Containers are fabric with no drainage holes. What are these larvae? (Sample submitted to the UMass Plant Diagnostic Laboratory.)

 A: These are the larvae of Anomala orientalis, which has been previously referred to as the Oriental beetle. This is a type of scarab beetle whose larvae are known as “white grubs”. Other scarab beetles in New England whose larvae are also known as white grubs include: Japanese beetle (Popillia japonica), European chafer (Amphimallon majale), and the Asiatic garden beetle (Maladera formosae). They can be differentiated by looking at the “raster pattern” or the arrangement of spines and the shape of the anal slit on the hind end of the grub/larva. A great resource for identifying white grub raster patterns can be found at https://ag.umass.edu/turf/fact-sheets/white-grub-identification. (A shout out and a thank you to Dr. Olga Kostromytska for confirming this!)
A: These are the larvae of Anomala orientalis, which has been previously referred to as the Oriental beetle. This is a type of scarab beetle whose larvae are known as “white grubs”. Other scarab beetles in New England whose larvae are also known as white grubs include: Japanese beetle (Popillia japonica), European chafer (Amphimallon majale), and the Asiatic garden beetle (Maladera formosae). They can be differentiated by looking at the “raster pattern” or the arrangement of spines and the shape of the anal slit on the hind end of the grub/larva. A great resource for identifying white grub raster patterns can be found at https://ag.umass.edu/turf/fact-sheets/white-grub-identification. (A shout out and a thank you to Dr. Olga Kostromytska for confirming this!)
Oriental beetle larvae can damage the roots of turf grasses as well as the roots of many nursery plants and small fruits, including containerized plants. Common ornamental hosts include hemlock, holly, rhododendron, azalea, juniper, and andromeda. Feeding by the larvae of this species on roots may lead to host plant yellowing or wilting of foliage. For more information about this insect, visit: https://ag.umass.edu/landscape/publications-resources/insect-mite-guide/anomala-exomala-orientalis.
Q: What are these? They are crawling all over the back of my woodshed. Thanks!
A: Both insects are two life stages of the same species. The insect on the left is an adult and the insect on the right is an immature boxelder bug (Boisea trivittata).
Boxelder bugs are known from eastern Canada throughout the eastern United States. They present the most nuisance when adults try to enter homes to overwinter. In the fall, they will gather in groups on the south sides of trees, buildings, and rock faces exposed to the sun. After they gather in large amounts, they fly together to homes or buildings, searching for a place to overwinter. In the spring, they leave their overwintering shelter and seek out expanding boxelder buds, where they lay eggs between late-April and early-May in the cracks and crevices of boxelder bark or surrounding sheltered areas. Eggs change in color from yellow to red as they mature. Nymphs are red when they emerge from hatched eggs and feed on fallen boxelder seeds. Up to two generations may occur per year, depending on local temperatures.
Boxelder bugs are primarily a nuisance household pest in the fall as adults seek shelter. They do not cause structural damage in buildings and do not bite people or pets. A way to discourage these insects from attempting to enter the home to overwinter is to be sure all window screens fit snugly and close any gaps or openings, even very small ones, that may allow entry into the home.
Generally, the boxelder bug is not a threatening pest to boxelder (Acer negundo). Foliage and twigs of female boxelders may be impacted slightly. This insect may cause foliar distortion, bronzing, or stippling. Boxelder bugs feed on seeds of female boxelders; remove female boxelder trees, if necessary, to reduce populations seeking overwintering shelters. Adults fly long distances, so removing nearby trees may not be entirely effective as additional hosts on neighboring properties may be located close enough to provide a population looking to overwinter.
Q: Do you know what this is? I am a landscaper in Franklin and Hampshire counties in MA and I found it on my pants.
A: Your image appears to be that of a deer ked, a type of blood-feeding parasitic fly in the Family Hippoboscidae (the louse flies). These flies feed primarily on deer and elk and other cervids. Winged adult deer keds emerge from puparia in September. They are active especially on warm afternoons and use vision to look for their hosts. They are attracted to large, dark, moving objects. Once males and females have found a host, both sexes drop their wings. Wingless keds are approximately 3/16th of an inch in length.
When on their host, they will bite multiple times to feed on blood. If they have a chance encounter with a human, they will bite people. However, this is relatively rare and the risk of encountering or interacting with a deer ked is, at this time, considered low. Some research suggests that deer keds may be able to transmit pathogens, but again the risk is thought to be low and research is ongoing. If a deer ked does bite a person, it only takes 15-25 minutes of feeding for it to become engorged with blood. The bite is barely noticeable, and at first there may be no sign that a person was bitten. In approximately three days, the bite location develops a hard, reddened welt. For up to 14-20 days following, the location of the bite may itch severely.
Deer keds are sometimes confused for ticks, because they are dorso-ventrally flattened (flattened from the top down) and brown in color. However, keds have 6 legs and adult ticks have 8. Keds also have a recognizable head, thorax, and abdomen (three body regions) whereas ticks only have two (a head and a body). More information about deer keds can be found at https://extension.unh.edu/sites/default/files/migrated_unmanaged_files/Resource004227_Rep6067.pdf.
Tawny Simisky, Extension Entomologist, UMass Extension Landscape, Nursery, & Urban Forestry Program
Garden Clippings Tips of the Month
December is the month to . . . .
-
Keep evergreen shrubs well-watered. Deep, late fall watering ensures that the roots of shrubs take up enough water to keep them hydrated during the winter. Broadleaf evergreens such as rhododendrons, holly, boxwood and mountain laurel can lose considerable amounts of moisture through their leaves during the winter months. This loss of water is mainly due to strong winds and sun, which can cause excessive foliage water loss while the roots are in frozen soil and unable to replace lost water. This results in desiccation and browning of plant tissue. To prevent desiccation injury, deeply water broadleaf evergreens in late fall if the soil is dry and continue watering on a regular basis until the ground freezes.
-
Protect tender evergreen shrubs from winter damage. Broadleaf evergreens are susceptible to dry cold winds which can cause desiccation of leaves. If they are exposed to cold dry winds, the leaves get desiccated and appear brown and dead in late winter and spring. Protect plants from drying winter winds by erecting wind breaks made of burlap attached to frames on the side facing the prevailing winds. Stakes can be placed about 12-18 inches away from the plant to support the burlap wind-buffering barrier.
-
Remove tall grass and vegetation around the bases of trees and large shrubs. Tall grasses and vegetation provide a protective cover for mice and voles where they can feed on the trunks and stems of trees and shrubs. They can girdle young trees and shrubs stems by chewing through the bark, causing serious damage. Cutting grasses and other vegetation short in late fall within 2 feet of young trees reduces protective cover for mice and voles that might feed on trunks and stems.
-
Use de-icing carefully to prevent plant damage. To prevent salt damage on plants, try coarse sand instead of salt to provide traction on driveways and sidewalks whenever possible. Also consider using non-sodium de-icing agents such as calcium chloride, magnesium chloride, potassium chloride, and calcium magnesium acetate (CMA). The non-sodium deicing agents are more expensive than sodium chloride but, if used as recommended, they do not cause harm to plants. To minimize plant injury due to de-icing salt, construct a physical barrier made of plastic, burlap or snow fencing to prevent splashing and salt spray along street edges.
-
Reduce watering and fertilizer applications to houseplants. During the months of cold temperatures and shorter days, the growth of most houseplants slows down. During this period, new growth is minimal. Reduce fertilizer applications and watering until spring when new growth resumes.
-
Protect poinsettias from cold. Poinsettias are very sensitive to cold temperatures. Protect them from freezing temperatures, especially when transporting them. When you bring them in the house, place in a light-filled room away from cold drafts. They do best in rooms between 55 and 65˚F at night and 65 to 70˚F during the day. Keep poinsettias away from cooler locations and avoid exposing them to temperatures below 50˚F.
-
Increase humidity in the house. During winter when furnaces are running, indoor air can be very dry with relative humidity sometimes goimg below 20%. Most houseplants prefer 40% to 50% humidity for normal healthy growth. Use a humidifier to increase humidity and move houseplants a little closer to each other to boost humidity. If a humidifier cannot be used, trays placed beneath plants and filled with constantly moist sand help increase humidity around the plants. Place pots with a saucer beneath each one on the tray with sand but avoid placing the pots directly on the wet sand.
-
Recycle the Christmas tree. Instead of throwing your used natural Christmas tree in the trash, consider recycling it on your property where the tree could decompose naturally while providing cover and food for wildlife and insects. Before you recycle, make sure the tree is free from ornaments and decorations. Do not recycle trees that have been sprayed with fake snow or treated with chemicals.
Geoffrey Njue, UMass Extension Sustainable Landscapes Specialist
Ecological Considerations of Establishing Oak Trees in the Urban Environment
Due largely to degraded growing environments that are inhospitable to many species, urban forests are often lacking in tree diversity, especially along streetscapes. While street trees constitute only a fraction of a typical community’s canopy cover, they represent the frontline to urban forests and are highly regarded for their public form and function. Data from both Maryland and Massachusetts illustrate the dearth of diversity among street tree populations in urban environments. Maples (Acer spp.) are the most common trees found along urban roadways, comprising upwards of 38% and 49% of street trees in Maryland and Massachusetts, respectively.
More recent research reflects similar patterns in other parts of the Northeast, including New Jersey, New York, and Pennsylvania, where maples are also found to be a dominant street tree genus. Cumming et al. (2006) posited that, based on the average size of these maples, the trees were likely planted several decades ago in response to the effects of the invasive Dutch elm disease (DED, Ophiostoma novo-ulmi) pathogen. Such a response to urban reforestation has the potential to be highly problematic considering further insect or disease outbreaks. For example, the Asian longhorned beetle (ALB, Anoplophora glabripennis) is regarded as one of the world’s 100 worst invasive species and the genus Acer is the known primary host for this insect pest (Dodds and Orwig 2011). Over 35,000 urban trees (mostly maple) were removed from the City of Worcester, MA, since the detection of ALB in 2008 (Quinn 2016).
As a result of occurrences like these, a growing number of urban foresters are becoming increasingly aware of urban forest uniformity. They are initiating management regimes that include intentional diversification of trees planted in urban forests to prevent the loss of community tree populations to a single, devastating pest species. In addition to expanding canopy cover as a strategy for climate change mitigation, addressing the urban forest diversity deficit is also recommended and implemented to increase resilience and adaptation.
Many urban foresters have placed increased emphasis on adhering to designated maximum percent urban tree compositions (%) aimed at substantially enhancing biodiversity. Santamour (1990) recommends that no more than 10% of a species, 20% of a genus, and 30% of a botanical family comprise an urban forest. Ryan and Bloniarz (2008) and Moll (1989) suggest no more than 10% of any one genus. Ball and Tyo (2016) recommend no more than 5% of any one genus. Generally, most urban foresters suggest a goal of no more than 5% to 15% of a species. When examining the occurrence of street trees like maples in Massachusetts and Maryland, we see that managers have not historically adhered to diversity recommendations.
In this article, we examine the presence and impact of oaks (Quercus spp.) in the urban environment to evaluate their role as street trees, since they are commonly and increasingly being used in urban greening and diversification efforts. We explore literature that focuses on two of the major implications of planting trees in human-dominated landscapes, with respect to diversity: infestation by invasive insects and nonnative diseases in monoculture ecosystems, and the attraction of wildlife to urban environments. These factors are especially pertinent to oaks, which are valued for their resilience and urban forest benefits including their potential to increase biodiversity by providing wildlife habitat and food. We consider the status (i.e., percent composition) of oaks in urban forests currently and touch upon how their distributions are expected to respond to future climate scenarios. Lastly, we use this information to provide suggestions for urban forestry professionals (e.g., community foresters and tree wardens) about where and when it may be appropriate to plant oak trees in their communities.
Approach
We searched peer-reviewed literature for information regarding general topics like urban biodiversity, wildlife, and insects and diseases. Four books (DeGraaf and Yamasaki 2001; Martin et al. 1951; McShea and Healy 2002; Tallamy 2009) were essential in gathering specific ecological information for oaks, especially regarding these trees as resources for wildlife. We reviewed other sources, including urban forestry trade journals, magazines, fact sheets, and conference proceedings, for recent data regarding urban forest composition, as well as trends in the field. Informal solicitation of information from urban forestry professionals also contributed to our knowledge of current goals and management practices. City foresters, arborists, tree wardens, and urban forestry faculty from Massachusetts and New York were contacted via e-mail. We asked questions of these authorities regarding planting habits and considerations related to oak trees in the urban environment and in response to invasive pest infestations. We also requested professional opinion on tree selection and performance, specifically regarding oaks, based on their anecdotal experience and street tree inventory data. Of the 14 urban forestry professionals contacted, seven responded. We rendered four of these comments most useful to cite within our work (see pers. comm. from Lefcourt, Bassuk, and Antonelli).
Oak Trees and Their Benefits to Wildlife
 Oak trees offer an abundance of benefits to wildlife. They are an important food source, with acorns rating at the top of the wildlife food list. Although acorns may not be preferred by many species, they are abundantly available, particularly in the winter when other food items are scarce. The storage of acorns as a future food resource for recovery during the winter months is common in caching species like squirrels and the acorn woodpecker (Melanerpes formicivorus). They are also important for bears during hyperphagia in the fall. Oaks play a significant role in shrub communities of the interior West, a region with limited food sources. In the east, oak trees have become increasingly significant for wildlife with the decline of both American chestnut (Castanea dentata) and American beech (Fagus grandifolia). Several wildlife species are known to consume acorns, from squirrels and other rodents to waterfowl and deer. Van Dersal (1940) found that upwards of 186 species of birds and mammals feed on oaks, while McShea and Healy (2002) assert that the distribution of many species corresponds with or relies on the range of oak trees. Martin et al. (1951) document 96 species known to consume acorns and estimate that acorns comprise anywhere from 5% to 25% of most species’ diets.
Oak trees offer an abundance of benefits to wildlife. They are an important food source, with acorns rating at the top of the wildlife food list. Although acorns may not be preferred by many species, they are abundantly available, particularly in the winter when other food items are scarce. The storage of acorns as a future food resource for recovery during the winter months is common in caching species like squirrels and the acorn woodpecker (Melanerpes formicivorus). They are also important for bears during hyperphagia in the fall. Oaks play a significant role in shrub communities of the interior West, a region with limited food sources. In the east, oak trees have become increasingly significant for wildlife with the decline of both American chestnut (Castanea dentata) and American beech (Fagus grandifolia). Several wildlife species are known to consume acorns, from squirrels and other rodents to waterfowl and deer. Van Dersal (1940) found that upwards of 186 species of birds and mammals feed on oaks, while McShea and Healy (2002) assert that the distribution of many species corresponds with or relies on the range of oak trees. Martin et al. (1951) document 96 species known to consume acorns and estimate that acorns comprise anywhere from 5% to 25% of most species’ diets.
In addition to acorn production, oak trees support wildlife species by providing cover and nesting sites, as well as supplies for these habitat elements. They host dozens of cavity-nesting bird species, including chickadees, bluebirds, woodpeckers, and owls. In some oak species, young trees retain their dried, dead leaves through winter. This phenomenon, known as marcescence, provides thermal cover and protection from predators. Perhaps one of the most notable yet underrated benefits of oaks is their significance to Lepidopteran species. The genus Quercus supports over 500 species of moths and butterflies, more than any other plant genus. In sustaining Lepidopteran species, oak trees add to the food sources available to birds.
Most importantly, oaks in the urban environment present natural opportunities for food and cover. According to DeGraaf and Yamasaki (2001), upwards of 193 wildlife species use red oak (Q. rubra) habitat in New England alone (Table 1). The authors generated natural history accounts for each species that breeds, winters, or resides in New England and created a species-habitat matrix to document relationships. They used 11 forest cover types that are predominant in New England to describe forest/wildlife habitat associations. Red oak habitat also includes associate species black oak (Q. velutina), scarlet oak (Q. coccinea), and chestnut oak (Q. montana), as well as hickory (Carya spp.) and red maple (A. rubrum). Of the 193 terrestrial vertebrates known to use red oak habitat, 23 prefer these stands over other cover types (Table 1). While planting more oaks in an urban setting would not provide the same degree of habit suitability available in natural forest systems, there is an opportunity to support variable taxa with tree species that are highly impactful for wildlife. Single street trees are less likely to achieve the high levels of biodiversity that natural systems support, but they may serve as corridors between urban parks, which could generate many of the same benefits.
Table 1. Taxa (number of species) that utilize Q. rubra habitat in New England. (Adapted from DeGraaf and Yamasaki 2001)
| taxa | Total | General use | preferred breeding habitat (PB) | Preferred non-breeding habitat (PNB) | PB & PNB |
|---|---|---|---|---|---|
| Amphibians | 15 | 13 | 2 | 0 | 0 |
| Reptiles | 16 | 15 | 1 | 0 | 0 |
| Birds | 114 | 101 | 12 | 3 | 2 |
| Mammals | 48 | 41 | 6 | 6 | 5 |
Oak Trees and Human-Wildlife Interactions
A variety of ecological effects may result from increasing the presence of oak trees in the urban environment, particularly regarding wildlife. Many wildlife species rely heavily on oaks for food and cover in rural forests, but this is also true for wildlife in urban areas. For example, caching species like jays and squirrels, which depend on acorn production and directly influence oak dispersal, can become prolific in the built environment as urban adapters. Since many Lepidopteran species require oak hosts, pollination and pollinator biodiversity and conservation can benefit from increased presence of trees within this genus. Residents may also benefit from access to nature and increased biodiversity in the built landscape. Surveys indicate urban residents enjoy and value wildlife, especially birds. Research has further demonstrated that residents are specifically interested in attracting wildlife to their backyards and that they gain personal satisfaction from feeding wildlife.
Despite the many wildlife-related benefits offered by oak trees, it is important to consider their potential disadvantages, especially from the human dimension standpoint. There are certainly costs that may be perceived concerning urban wildlife. Negative perceptions of urban wildlife are often attributed to species for their numbers or for potential threats that they pose to people and property. Generally, these include damage to plants and structures, droppings, threats to pets, annoyance to humans, animal bites, and transmission of disease. Thus, public attitudes are largely influenced by the types of contact and experiences residents have with urban wildlife. Urban wildlife that are frequently dubbed problematic include small and large mammals, especially carnivores. Where these species create conflict, measures to avoid, deter, or even remove them are often instituted.
The concept of “wildlife acceptance capacity” (WAC) factors heavily into human perceptions of wildlife. Decker and Purdy (1988) defined WAC as the maximum wildlife population level in an area that is acceptable to people. There can be significant variation in WAC among different individuals or stakeholder groups, and tolerance may change over time. WAC is strongly influenced by how people perceive risks associated with wildlife, such as threats to health and safety.
Increasing, oak trees in the urban environment could potentially exacerbate human-wildlife conflict if doing so contributes to meeting or exceeding the WAC for a species. As previously described, many wildlife species rely on the acorns dropped by oaks as a staple of their diet. Some of these species, such as deer and small mammals, are considered a nuisance when they occur in high densities. Planting more oaks could increase the abundance of wildlife species that consume acorns, thereby approaching or exceeding the WAC for these species. Similarly, with expanded populations of prey species, there may be opportunity for an increase in predator populations as their food sources become more abundant. For example, coyotes (Canis latrans), which commonly utilize urban areas, feed on small mammals, whose distribution would likely be affected by oak trees. More importantly, the coyote is known as a nuisance species, occasionally posing threats to humans and their pets.
When examining the possible ramifications of oak trees in the urban environment, an important relationship evidently exists between oak mast, small mammals, insect vectors, and Lyme disease, caused by Borrelia burgdorferi. Both the population numbers and behavioral habits of white-footed mice (Peromyscus leucopus) and white-tailed deer (Odocoileus virginianus) are strongly influenced by acorn production, with mast years yielding increased numbers of both species within oak stands. Mouse densities tend to increase following mast production, while deer respond to mast years by foraging in forests dominated by oaks over other stand types. Of the species known to host larval deer ticks (Ixodes scapularis), scientists have concluded that the white-footed mouse is the host most likely to transmit the Lyme disease bacterium.
White-tailed deer are notorious for harboring adult deer ticks, which are ungulate specialists. Consequently, the location of deer in the fall determines where larval deer ticks, produced by adult deer ticks, occur in the landscape. This causes heavy concentrations of larval ticks in forests dominated by oak trees during mast years, when deer exploit the abundance of acorns. As a result, larval ticks co-occur in time and space with white-footed mice, increasing the likelihood for transmission of Lyme disease. Complexities to this dynamic relationship are introduced when land use and human behavior are considered. Increasing development and incidents of Lyme disease, especially in the Northeast, pose risks to human health, with recent research indicating that threats are not restricted to suburban and natural settings. These findings suggest that caution should be exhibited in urban green space and residential landscape design.
Planting Oak Trees as a Response to Invasive Insects
Invasive insect pests have the capacity to cause wide-scale disturbance and destruction in natural and urban ecosystems. Many native tree species abundant in Northeast forests and historically preferred as street trees have experienced significant decline due to nonnative insect invasions. While oaks are not pest-free, there are far fewer accounts of such large scale, dramatic losses to invasive insects compared to other landscape trees. For example, the emerald ash borer (EAB, Agrilus planipennis), an introduced pest from Asia, has killed tens of millions of ash trees in rural and urban areas throughout the range of Fraxinus spp. in North America. In southeastern Michigan, it has caused virtually 100% mortality of ash species. In addition to ecological ramifications, infestations at this scale are also damaging to the economy. Based on stumpage value alone, loss of the ash resource in Michigan is projected to exceed $1.7 billion. EAB is but one of many non-native pests to negatively affect ecosystems and economies in the U.S.
Considered one of the most destructive wood borers to invade the region in recent years, Asian longhorned beetle (ALB) is native to China and Korea. The pest was first detected in the U.S. during 1996, where it was introduced to New York City. Most recently, it has been found in central and eastern Massachusetts, primarily in the city of Worcester. Susceptible tree species include – but are not limited to – maple, horse chestnut (Aesculus spp.), birch (Betula spp.), ash, poplar (Populus spp.), willow (Salix spp.), and elm (Ulmus spp.). ALB infests healthy host plants and proceeds to actively feed and move throughout the tree via extensive larval tunneling. In as little as one or two years, repeated attacks in the vascular tissue and structural weakness can lead to host death; however, plants may live substantially longer. Specifically, the beetle targets sugar maple (A. saccharum), red maple, and Norway maple. This is particularly problematic in a region like New England, where both sugar maple and red maple are prominent hardwood species, and where Norway maple was extensively planted in urban settings, not only in response to the devastating effects associated with DED, but also for its structural resistance to failure under intense weather events.
While oaks appear to be plausible alternatives to planting more maples, since they are potentially unsuitable hosts for ALB, the genus is not immune to infestation from invasive insects. Following accidental introduction to Massachusetts in the 1860s, the spongy moth (Lymantria dispar, form. gypsy moth) has spread throughout the Northeast, where it causes considerable damage to numerous tree species, but especially oak stands in New England. Spongy moth defoliation has both direct and indirect, as well as short- and long-term, implications for oak communities. In addition to tree decline and mortality, wide scale defoliation can cause changes in light, temperature, and moisture regimes, along with the alteration of nutrient cycles. Other ecological consequences include stand patchiness, sporadic masting, and modifications in successional patterns and watershed characteristics. Impacts such as these have the potential to consequently affect wildlife distribution patterns. Stress from spongy moth defoliation also impacts acorn production, resulting in the decrease or elimination of this important wildlife food source. In addition to ravaging Northeast forests, high numbers of spongy moth are even more problematic in urban settings, where urticating hairs and frass production cause human health concerns.
The likelihood of spongy moth outbreaks depends to some extent on oak mast. Mast years occur every 2 to 5 years, resulting in large acorn crops. Acorns are a dominant dietary component for white-footed mice, which are important predators of spongy moth pupae. In years between oak mast, mouse densities are reduced, yielding increased numbers of spongy moth and initiating potential for outbreaks of this invasive pest (Jones et al. 1998). Some research and anecdotal evidence indicate that spongy moth caterpillars prefer white oak (Q. alba) over other species of the genus, with variation in results and location. It is likely that a combination of foliar characteristics influences oak susceptibility to infestation.
Planting Oak Trees as a Response to Invasive Pathogens
In addition to insects of importance, tree populations may also succumb to invasive pathogens, which can have similarly detrimental effects (Loo 2008; Lovett et al. 2006). This is especially true in the urban environment, where low levels of diversity in the urban forest limit resiliency to the spread of insects and disease. A classic example of this involves the renowned American elm (U. americana). Dutch elm disease (DED), which was introduced from Europe on shipments of unpeeled veneer logs, is regarded as one of the most devastating shade tree diseases in the U.S. The fungal pathogen that causes DED is transported by elm bark beetles and transmitted by root grafts, resulting in tree wilting as well as yellowing and browning of leaves. Following infection, these symptoms continue throughout the tree’s crown, resulting in eventual host-plant death. Dutch elm disease was first detected in 1930 and spread nationwide by 1977, decimating populations of elm, a fast-growing, stress-tolerant hardwood species that once prolifically lined streets, parks, and landscapes throughout North American communities.
Similarly, chestnut blight, caused by Cryphonectria parasitica, is a fungal disease that pervaded eastern hardwood forests at a rate of 24 miles per year. Cankers from the disease were sighted in New York City in 1904. First detected on shade trees, chestnut blight contaminated nearly all adult chestnut trees by the 1950s. Like the elms, the chestnut fell from its former status as a major component of eastern hardwood forests in the U.S. Consequently, wildlife species were dramatically impacted with the loss of this significant mast crop, placing more reliance on other food sources like acorns. While oak trees in the Northeast are essentially unaffected by DED and chestnut blight, the risk of infection from other pathogens of importance remains.
Oak wilt, caused by the fungus Ceratocystis fagacearum, is a serious pathogen relative to oaks. First discovered in Wisconsin in 1942, it has since caused the dramatic and rapid death of red oak throughout parts of the mid-west and in many western states. In Wisconsin, localized areas of the state have experienced loss of over 50% of oak populations due to the presence of this pathogen. In the Northeast, oak wilt threatens oak trees constituting the oak-hickory forest types. Infection is indicated by sudden wilting and death of host tree foliage and may sometimes manifest as fungal mats that form below the bark. The disease is most prominently dispersed through root grafts but may be vectored by insects, namely sap-feeding beetles (family Nitidulidae) and bark beetles (family Curculionidae) that carry fungal spores to new hosts.
The origin of oak wilt is unknown, though it is suspected that native populations of the pathogen may be found in Mexico, Central America, and northern South America. In the U.S., oak wilt ranges from the mid-west, south to Texas, and east to Pennsylvania, with localized detections in New York. Red oak (Erythrobalanus) species can succumb within weeks of infection and are more susceptible than white oak (Leucobalanus) species, which may take several years to experience mortality. Though oak wilt is likely a greater threat to rural forests outside of the region, potential range expansion of oak wilt, along with differences in oak species resistance, have direct implications for urban forests in the Northeast. With pest and disease outbreaks potentially on the horizon, careful thought to the selection of less susceptible oaks planted with consideration for street tree diversity can help to maintain an urban forest that is more robust to invasion.
Climate Change
Along with resistance to specific urban pests, oak trees in the Northeast are anticipated to fare well under future climate change forecast models, where forest range types shift increasingly northward. As global temperatures continue to rise, oaks adapted to more southerly climates could exhibit tolerance and even expansion under warmer conditions. A comparison of climate change scenarios and resulting suitable habitat indicated that the oak-hickory forest type is expected to expand north, especially under higher emissions scenarios. Coniferous forests of the Northeast and the maple-birch-beech (Fagus spp.) forest type, in contrast, are predicted to contract.
Planting southerly oak species in specific locales of the Northeast could assist the migration of species northward, giving them a head start in anticipation of shifting weather patterns and an extended growing season. This seems to be on the radar for some urban forestry professionals. In the city of Cambridge, Massachusetts, more trees from the Mid-Atlantic area, especially oaks, are being selected and installed due to the current and future impacts of climate change. The hope is that these trees are more resilient to drought conditions experienced during the summer months but can survive through harsh winter conditions, as well. It is important to note that insect and pathogen pressure is likely to increase with climatic warming, though it is unknown to what extent oaks will be affected as southern species move north.
Conclusions
In the context of diversity, invasion susceptibility, and wildlife in urban environments, oak trees have the potential to improve the urban forest. Urban forests infamously lack biodiversity, especially regarding street tree genera, where near monocultures threaten the health and resilience of urban tree populations plagued by diversity deficits. While some city departments and foresters still strive to find what Bassuk (1990) refers to as the "perfect urban tree," many urban foresters have started to recognize the importance of increasing the diversity of the trees that they select for planting. One of the main drivers behind this recent management strategy shift in the northeastern U.S. has been in relation to the catastrophic loss of trees from exotic insect and disease infestations, including ALB, EAB and DED. Oak trees are not known hosts to these high-profile insect or disease pests, making them especially favored candidates for urban tree planting efforts.
While increasing oak trees may appear advantageous in some scenarios, there are drawbacks to consider. We have indicated that augmentation of oaks in the urban environment may become less desirable if wildlife populations demonstrably increase and exceed acceptable citizen thresholds. Acorns can also cause oaks to be viewed negatively in situations where they cause direct discomfort to urban residents. During mast years, mature oak trees are notoriously associated with a “messiness” caused by heavy acorn production, where sidewalk conditions have been compared to walking on ball bearings. The potential for increased prevalence of zoonotic diseases and susceptibility to spongy moth infestation, as well as the threat of infestation from the lethal oak wilt pathogen, are also important factors to consider. With these possible ramifications in mind, we might be wary of creating another monoculture situation if aiming to improve diversity with species from this genus. For example, though not evenly distributed statewide, an average of 15% of Massachusetts street trees are comprised of oaks. Furthermore, we see that oaks represent 22% of trees replanted in response to ALB invasion in the city of Worcester.
Based on the details of our synthesis, we suggest that planting oak trees will have the greatest positive impact in specific communities where the genus is not overly represented (i.e., does not already exceed 10%–20%) in the local street tree population. Expansion of oak populations in urban forests might be considered carefully, and perhaps avoided, in communities where there are concerns for infestation by spongy moth, oak wilt infection, and/or perhaps even the transmission of tickborne diseases. Species-specific planting decisions may help to mitigate potential effects of pests and pathogens, as well as wildlife. In anticipation of human-wildlife conflict or negative reactions to acorn abundance in residential areas, oak species that are particularly attractive to wildlife could be planted along the outskirts of urban centers or carefully incorporated into urban parks. This may help mitigate some of the issues surrounding oak mast. Residents may be notified that, during mast years, there will likely be increased abundance of mammals and possibly tickborne diseases. During intervening years, when acorn production is minimal, threats to invasion by spongy moth might be expected. Finally, the use of oak trees in efforts to promote urban forest diversity offers the potential for increasing urban forest resilience as climate change progresses. It is ever important to reiterate the urban forestry mantra “right tree, right place” when considering which species to plant, as suitability, adaptability, and potential liabilities exist with each management decision.
Richard W. Harper, Ph.D., Extension Associate Professor of Urban & Community Forestry, Dept of Environmental Conservation, UMass Amherst; Tierney Bocsi, University of Wisconsin Madison; Paige S. Warren, University of Massachusetts Amherst; and Stephen DeStefano, U. S. Geological Survey, Massachusetts Cooperative Fish and Wildlife Research Unit, University of Massachusetts Amherst
Upcoming Events
For details and registration options for these upcoming events, go to the UMass Extension Landscape, Nursery, and Urban Forestry Program Upcoming Events Page.
-
Jan 30 - Winter Workshop: Pollinator Topics, live webinar 9:30 am to 1:30 pm. Credits available: 1 pesticide contact hour for categories 29, 35, 36, 40, or Applicators License. Association credits: 1 MCA, 1 MCLP, and 1 MCH. ISA credits requested.
-
Feb 6 - Winter Workshop: Tree and Shrub Insect Topics, live webinar 9:30 am to 12:15 pm. Credits available: 2 pesticide contact hours for categories 29, 35, 36, 40, or Applicators License. Association credits: 1 MCA, 1 MCLP, and 1 MCH. ISA, SAF, and CFE credits requested.
Additional Resources
For detailed reports on growing conditions and pest activity – Check out the Landscape Message
For professional turf managers - Check out our Turf Management Updates
For commercial growers of greenhouse crops and flowers - Check out the New England Greenhouse Update website
For home gardeners and garden retailers - Check out our home lawn and garden resources
TickTalk webinars - To view recordings of past webinars in this series, go to: https://ag.umass.edu/landscape/education-events/ticktalk-with-tickreport-webinars
Diagnostic Services
Landscape and Turf Problem Diagnostics - The UMass Plant Diagnostic Lab is accepting plant disease, insect pest and invasive plant/weed samples. By mail is preferred, but clients who would like to hand-deliver samples may do so by leaving them in the bin marked "Diagnostic Lab Samples" near the back door of French Hall. The lab serves commercial landscape contractors, turf managers, arborists, nurseries and other green industry professionals. It provides woody plant and turf disease analysis, woody plant and turf insect identification, turfgrass identification, weed identification, and offers a report of pest management strategies that are research based, economically sound and environmentally appropriate for the situation. Accurate diagnosis for a turf or landscape problem can often eliminate or reduce the need for pesticide use. See our website for instructions on sample submission and for a sample submission form at http://ag.umass.edu/diagnostics.
Soil and Plant Nutrient Testing - The lab is accepting orders for Routine Soil Analysis (including optional Organic Matter, Soluble Salts, and Nitrate testing), Particle Size Analysis, Pre-Sidedress Nitrate (PSNT), Total Sorbed Metals, and Soilless Media (no other types of soil analyses available at this time). Testing services are available to all. The lab provides test results and recommendations that lead to the wise and economical use of soils and soil amendments. For updates and order forms, visit the UMass Soil and Plant Nutrient Testing Laboratory web site.
Tick Testing - The UMass Center for Agriculture, Food, and the Environment provides a list of potential tick identification and testing options at: https://ag.umass.edu/resources/tick-testing-resources.


