To print this issue, either press CTRL/CMD + P or right click on the page and choose Print from the pop-up menu.
Click on images to enlarge.

Thank you to our 2024 Sponsors!
Crop Conditions
Fields are drying out and folks are out plowing, discing, and putting the first pea seeds and brassica, lettuce, scallion, beet, and chard transplants into the ground. Tunnels continue to be turned over from winter to summer crops. Garlic plants are growing fast now, and for those using inorganic forms of nitrogen, it’s time to fertilize! Research from Cornell Extension has shown that the ideal fertility plan for garlic is to apply 50 lbs/A N in the spring. The plants will take up all the N that they need by the end of May. Most N sources available to organic growers need to be processed by soil microbes in order to become available to plants, which takes time in cold soils, so organic growers will likely need to continue relying on fall-applied N, and should apply up to 100 lbs/A N.
Pest Alerts
 Late blight: A late blight outbreak was observed on high tunnel tomatoes in central NY last week. We encourage growers to be extra vigilant in scouting for late blight in high tunnel tomato crops and seedling flats right now and remain vigilant with scouting as the field season starts. If growers observe late blight on potatoes or tomatoes they should bag and remove infected plants (send to the landfill) or otherwise destroy them—do not compost infected tissue as it can reinfect surrounding plantings. If you suspect late blight in MA, please contact us at umassveg@umass.edu or 413-577-3976. Protectant fungicides like copper and chlorothalonil must be applied before disease becomes established. Systemic fungicides like Ridomil and Orondis have stronger activity but are still best used when disease levels are low. Fungicide applications for late blight are not yet recommended for MA growers.
Late blight: A late blight outbreak was observed on high tunnel tomatoes in central NY last week. We encourage growers to be extra vigilant in scouting for late blight in high tunnel tomato crops and seedling flats right now and remain vigilant with scouting as the field season starts. If growers observe late blight on potatoes or tomatoes they should bag and remove infected plants (send to the landfill) or otherwise destroy them—do not compost infected tissue as it can reinfect surrounding plantings. If you suspect late blight in MA, please contact us at umassveg@umass.edu or 413-577-3976. Protectant fungicides like copper and chlorothalonil must be applied before disease becomes established. Systemic fungicides like Ridomil and Orondis have stronger activity but are still best used when disease levels are low. Fungicide applications for late blight are not yet recommended for MA growers.

 Allium leafminer emergence has begun in Plymouth, Bristol, Barnstable, and Dukes counties, and is expected to begin in the next week or so in most of the rest of the state. Adult flies will emerge from fields that had infested crops last year and fly to find new allium hosts (e.g., scallions, perennial chives, overwintered onions, wild allium species). Flies are active for 5-6 weeks (usually until approximately the end of May). The maggots will tunnel down to the base of the plant, and the damage can result in secondary rots. The presence of maggots or pupae within the plants makes crops unmarketable. When emergence begins in your area, start scouting any unprotected allium crops for straight lines of small (~2 mm diameter), white oviposition marks. These marks are often found at the tips of leaves. Adults can also be trapped using yellow sticky cards placed within the crop. Adults have a distinctive yellow-orange spot at the tops of their heads (see photo). If ALM flies are not likely to be emerging from the soil around your spring allium crop (i.e., there’s no history of infestation in the field), you can cover crops with row cover or insect netting to exclude flies. Conventional growers can apply dinotefuran (e.g. Scorpion, Venom), cyantraniliprole (Exirel), or spinetoram (Radiant), starting up to 2 weeks after feeding/oviposition marks are detected. All of the above materials have systemic or translaminar activity and will be effective against larvae. Include a surfactant to limit runoff and maximize uptake, unless labels prevent it. Organic growers should apply Entrust at 6 oz/A + M-Pede at a 1-1.5% v/v solution, two times, 2-4 weeks after first detecting ALM in a crop.
Allium leafminer emergence has begun in Plymouth, Bristol, Barnstable, and Dukes counties, and is expected to begin in the next week or so in most of the rest of the state. Adult flies will emerge from fields that had infested crops last year and fly to find new allium hosts (e.g., scallions, perennial chives, overwintered onions, wild allium species). Flies are active for 5-6 weeks (usually until approximately the end of May). The maggots will tunnel down to the base of the plant, and the damage can result in secondary rots. The presence of maggots or pupae within the plants makes crops unmarketable. When emergence begins in your area, start scouting any unprotected allium crops for straight lines of small (~2 mm diameter), white oviposition marks. These marks are often found at the tips of leaves. Adults can also be trapped using yellow sticky cards placed within the crop. Adults have a distinctive yellow-orange spot at the tops of their heads (see photo). If ALM flies are not likely to be emerging from the soil around your spring allium crop (i.e., there’s no history of infestation in the field), you can cover crops with row cover or insect netting to exclude flies. Conventional growers can apply dinotefuran (e.g. Scorpion, Venom), cyantraniliprole (Exirel), or spinetoram (Radiant), starting up to 2 weeks after feeding/oviposition marks are detected. All of the above materials have systemic or translaminar activity and will be effective against larvae. Include a surfactant to limit runoff and maximize uptake, unless labels prevent it. Organic growers should apply Entrust at 6 oz/A + M-Pede at a 1-1.5% v/v solution, two times, 2-4 weeks after first detecting ALM in a crop.
Aphids in high tunnel crops: As folks are finishing up high tunnel crops and turning tunnels over, we’ve been getting lots of questions about aphid control and whether aphids in winter crops will carry over to summer crops. It depends on the aphid species – some species have a wide host range  while others are more specific. See the article in this issue for pointers on identifying aphid species (reach out to us for help!) and information about establishing a biocontrol program.
while others are more specific. See the article in this issue for pointers on identifying aphid species (reach out to us for help!) and information about establishing a biocontrol program.
Lesion nematodes were identified in a spring high tunnel lettuce planting last week. The lettuce plants were turning brown and rotting from the base up, and the grower reported similar symptoms in kale planted in the tunnel last fall. Several very common soil-borne fungal pathogens can cause identical symptoms to these, so getting an accurate diagnosis from the UMass Plant Diagnostic Lab is critical. Lesion nematodes have a very wide host range including all vegetable crops, minimizing the efficacy of crop rotation as a management strategy. The invasion of the nematode into the root creates necrotic areas that vary from individual lesions to necrosis of the entire root system, and the damage can cause entry points for secondary rot pathogens. Management options include biofumigation with green manures (e.g. sudangrass hybrids, sorghum-sudangrass, brown mustard), pre-plant fumigation with conventional soil fumigants, anaerobic soil disinfestation, and/or the removal and replacement of the top 2 feet of soil.
Contact Us
Contact the UMass Extension Vegetable Program with your farm-related questions, any time of the year. We always do our best to respond to all inquiries.
Vegetable Program: 413-577-3976, umassveg@umass.edu
Staff Directory: https://ag.umass.edu/vegetable/faculty-staff
Home Gardeners: Please contact the UMass GreenInfo Help Line with home gardening and homesteading questions, at greeninfo@umext.umass.edu.
Be on the Lookout for Spring Maggot Pests
There are three maggot fly pests that are active on Massachusetts vegetable farms in early spring: seed corn maggot, cabbage maggot, and onion maggot. The emergence of adult flies from pupae that overwintered in the soil can be predicted using growing degree days (GDDs)1 with a base temperature of 40°F. The base temperature for monitoring the emergence of maggot flies is lower than the base temperature for many other vegetable pests because they are active at fairly cool temperatures early in the spring. These three maggot fly pests emerge and reach peak flight at different times throughout the spring (Table 1) and infest different crops. Seed corn maggot reaches peak flight earliest in the spring and has a host range of over 30 crops (including alliums and brassicas). This maggot is a common reason for poor germination of peas or small plantings of sweet corn. Cabbage and onion maggots are host-specific, attacking brassicas and alliums, respectively.
1Growing degrees days (GDD) are the number of degrees that the average daily temperature exceeds a base temperature at which a particular organism is dormant. GDD = ((Tmax + Tmin)/2) - Tbase. If the average temperature for a day is lower than the base temperature, then no GDD accumulate. GDD accumulate daily, starting on a specific date, by adding each day’s total GDD to the previous tally.
| Seed Corn Maggot |
Cabbage Maggot | Onion Maggot | |
|---|---|---|---|
|
Host |
40 different plants, large germinating seeds, seedlings (including alluim and brassica!) | Brassicas | Alliums |
| First Peak Flight | 360 GDD base 40°F | 452 GDD base 40°F | 735 GDD base 40°F |
|
Adult |
Small: ~ 3mm, 3 stripes on the thorax | Medium: ~5mm, 2 stripes on the thorax. | Large: ~6mm. |
| Eggs | Hatch in 2-4 days | Hatch in 7-10 days | Hatch in 2-5 days |
| Larvae (Maggot) | Active for 3 wks | Active for 2-4 wks | Active for 2-3 wks |
| Pupae | In soil for 1-2 wks before next gen adults emerge (last gen pupae overwinter) | In soil for 2-3 wks before next gen adults emerge (last gen pupae overwinter) | In soil for 3-4 wks before next gen adults emerge (last gen pupae overwinter) |
| Notes | Short, 21-day lifecycle. 3 gen per year. Usually only spring gen is damaging. | Long, 60-day lifecycle. 4 gen per year. Spring and Fall gen most damaging. | Medium, 30-day lifecycle. 3 gen per year. Usually only spring gen is damaging. |
| Location | GDD (base 40°F) |
|---|---|
| Western MA | |
| Deerfield | 220 |
| Westfield Arpt | 269 |
| Chicopee Falls | 274 |
| South Deerfield | 250 |
| Central MA | |
| Leominster | 225 |
| Northbridge | 249 |
| Worcester | 252 |
| Eastern MA | |
| Bolton | 242 |
| Stow | 238 |
| Lawrence Arpt | 230 |
| Ipswich | 288 |
| East Bridgewater | 303 |
| Boston | 284 |
| Providence, RI | 311 |
Seed corn, cabbage, and onion maggots share many characteristics. There are three to four generations of each of these pests per year. They prefer cooler temperatures, so the spring and fall generations are typically worse than the mid-summer generation(s). The adult flies of all three species emerge from pupae that overwintered in fields where a host crop was the previous fall. Adults emerge from the soil, mate, and then search for a host plant. Eggs are laid at the bases of host plants or on emerging seedlings. The resulting larvae will feed on host roots, causing the plants to collapse, or, in the case of seed corn maggot, kill seedlings before they emerge. All three flies are attracted to decomposing organic matter, and infestations are often worse in manured, cover-cropped, or composted fields where organic matter is still breaking down. Several consecutive days of soil temperatures above 95°F can kill the larvae. All three maggot fly adults are similar in appearance (small, gray, humpbacked, housefly-like) and size (5-7mm).
Preventive measures are generally most effective for managing these pests, as chemical treatment options are limited. These may include using floating row covers or insect netting to protect plants from egg-laying adults, delaying planting susceptible crops until the first emergence has largely passed, or waiting to plant until soil temperatures are high enough to kill larvae. Most labeled pesticides for maggots are labeled only for use pre-plant, at the time of planting or seeding, or immediately after setting transplants. Use pre- or at-plant treatments where damaging populations are expected, such as in fields with high organic matter or a history of infestations. Scouting for adults and eggs can help you understand infestation levels and inform management decisions in future plantings.
Below is more information about each maggot pest, including additional scouting and management recommendations:
Seed corn maggot (Delia platura): Seed corn maggot adults have likely begun emerging in many fields in MA, with peak flight expected within the next week in eastern MA and within the next few weeks in central and western MA. Emerging flies will lay eggs on the soil surface. Hatching larvae will burrow into the soil in search of food and penetrate seeds as the seed coat splits open, killing the seeds before germination and causing poor stand.
Where possible, delay planting for several weeks in the spring after a cover crop is incorporated to allow organic matter to break down. Warmer soils with more decomposed organic matter will mean fewer problems with seed corn maggot. Floating row cover is not as effective in managing seed corn maggot because this pest has many hosts and could have overwintered in virtually any field on your farm. If you cover plants in an infested field, the adults will emerge under the row cover. Organic fertilizers containing seed meals can attract this pest. Other pests and diseases, including wireworms and damping off, can also prevent seedlings from emerging; check for maggots and feeding tunnels inside seeds or stems to confirm what pest you’re dealing with. Plant shallowly to promote rapid seed emergence. Among bean varieties, those with a dark seed coat sustain less injury than white varieties. Preventative chemical treatments include commercially applied systemic seed treatments and in-furrow applications of insecticides. Rescue treatments are not effective. If there is enough damage to warrant replanting, wait at least 5 days if maggots are a quarter inch long; if they are smaller than that, wait at least 10 days to make sure they have pupated and will not damage the new seeds.
 Cabbage maggot (Delia radicum): Cabbage maggot flies are either nearing or just past first emergence across the state depending on the accumulated GDDs in your area (see Table 2 on previous page). A good indicator of the first cabbage maggot peak flight is blooming of the common roadside weed yellow rocket or wintercress (Barbarea vulgaris). Row covers can be very effective in the spring against this pest as long as crops are rotated into fields without a history of recent infestation since, as with seed corn maggot, if there are pupae in the soil from the previous season the adults will emerge under the row covers.
Cabbage maggot (Delia radicum): Cabbage maggot flies are either nearing or just past first emergence across the state depending on the accumulated GDDs in your area (see Table 2 on previous page). A good indicator of the first cabbage maggot peak flight is blooming of the common roadside weed yellow rocket or wintercress (Barbarea vulgaris). Row covers can be very effective in the spring against this pest as long as crops are rotated into fields without a history of recent infestation since, as with seed corn maggot, if there are pupae in the soil from the previous season the adults will emerge under the row covers.
Cabbage maggot larvae feed on brassica crop roots, causing stunting, wilting, and plant death. In brassica roots crops like radishes, turnips, and rutabaga, cabbage maggot tunneling renders the crops unmarketable. Inspecting the roots of symptomatic plants may reveal the legless, white maggots, brown oblong pupae, and/or tunnels from maggot feeding.
 There can be up to 4 generations of cabbage maggot per year. There is a model for tracking the emergence of cabbage maggot on the Network for Environmental and Weather Applications (NEWA) website: NEWA Cabbage Maggot Model. On the left-hand side of the page, choose a weather station that is close to you. When GDDs indicate peak flight (452 GDDs), or when adult flies are found on sticky cards placed in the field, begin scouting
There can be up to 4 generations of cabbage maggot per year. There is a model for tracking the emergence of cabbage maggot on the Network for Environmental and Weather Applications (NEWA) website: NEWA Cabbage Maggot Model. On the left-hand side of the page, choose a weather station that is close to you. When GDDs indicate peak flight (452 GDDs), or when adult flies are found on sticky cards placed in the field, begin scouting
every 3-5 days. A pencil point or knife helps stir the soil to look for eggs. Field scout by checking 25 plants, in groups of 2-5 plants, scattered around the field. Eggs may be more abundant in wetter areas of the field. There are no chemical treatment options at this stage, but again, scouting will help you determine the extent of infestations and whether an at-plant treatment should be applied in subsequent plantings.
There are more pesticide options for control of cabbage maggot than the other maggot pests. Available products can be found in the appropriate crops’ insect control section of the New England Vegetable Management Guide. Coragen (chlorantraniliprole) or Verimark (cyantraniliprole) may be applied at planting and Radiant (spinetoram) or Entrust (spinosad) may be applied to leafy (not root) brassicas at planting and in up to two additional applications to  seedlings. Other management tips include:
seedlings. Other management tips include:
- Delay planting until after first peak flight (usually mid-May, depending on GDDs) or when soil temperatures are high enough to kill eggs (95°F). Planting in late-May into June is generally safer than in the first half of May.
- Cultivate vigorous brassica crops so that soil is brought up around the stem to encourage adventitious root formation. This can help compensate for root loss if maggots are present.
- Natural enemies: naturally occurring soil-dwelling beetles, including carabid ground beetles and staphylinid rove beetles, feed on cabbage maggot eggs, larvae, and pupae and can cause high levels of mortality. One staphylinid species, Aleochara bilineata, also parasitizes maggot larvae and has been shown to respond to chemicals given off by plants that suffer maggot damage. Because these soil-inhabiting beetles are susceptible to insecticides, broadcast soil insecticide treatments should be avoided. Other natural enemies include parasitic wasps and predatory mites.
- Nematodes for biological control: soil application of the entomopathogenic nematode Steinernema feltiae has shown efficacy against cabbage maggot in trials even at low soil temperatures (50°F/10°C). Apply by suspending juvenile nematodes in water and treating transplants prior to setting in the field (as a spray or soaking drench), or in transplant water used in a water wheel transplanter, or a combination of pre-plant and post-plant applications. Post-plant treatments are likely necessary if maggot flight begins more than 1 week after transplanting. Rates of 100,000 to 125,000 infective juveniles per transplant have been shown to be needed to achieve reduction in damage. Nematodes need a moist soil environment to survive.
 Onion Maggot (Delia antiqua): This pest begins its flight when cabbage maggots are at peak flight; yellow rocket bloom is an indicator of the beginning of onion maggot flight. NEWA also has a pest forecast model for onion maggot emergence: NEWA Onion Maggot Model. Delaying planting is not a practical method of avoiding this pest because onions are typically planted very early in the spring and are in the ground about a month before the onion maggot becomes a problem. In onions, newly hatched larvae crawl behind the leaf sheath, enter the bulb, and feed on the roots, stem, and developing bulb. Feeding damage also allows for entry of soft rot pathogens. Some tips specific to managing onion maggot include:
Onion Maggot (Delia antiqua): This pest begins its flight when cabbage maggots are at peak flight; yellow rocket bloom is an indicator of the beginning of onion maggot flight. NEWA also has a pest forecast model for onion maggot emergence: NEWA Onion Maggot Model. Delaying planting is not a practical method of avoiding this pest because onions are typically planted very early in the spring and are in the ground about a month before the onion maggot becomes a problem. In onions, newly hatched larvae crawl behind the leaf sheath, enter the bulb, and feed on the roots, stem, and developing bulb. Feeding damage also allows for entry of soft rot pathogens. Some tips specific to managing onion maggot include:
- Minimize mechanical and chemical damage to onions throughout the season. Onion root systems are not as hardy as brassicas, so hilling them will not encourage more root production or recovery from root feeding. Hilling leeks can be a recommended practice for developing longer stalks though.
- Gather culled bulbs into deep piles as opposed to deep plowing or harrowing after harvest. This will limit fly reproduction to the surface layers of the cull pile.
- Naturally occurring fungal diseases occasionally will reduce onion maggot numbers, particularly when flies are abundant and relative humidity is high. During a fungal epidemic, dead flies can be seen clinging to the highest parts of plants along field edges.
- As with cabbage maggot, predaceous ground beetles and entomopathogenic nematodes may help reduce maggot numbers. Avoid broadcast insecticide treatments to protect beneficial insects and follow nematode applications recommendations above.
See the New England Vegetable Management Guide for pesticide options.
--UMass Vegetable Program
Aphid Biocontrol in High Tunnels
There are both chemical and biological control options for managing aphids in high tunnels; this article will outline biological control and the aphid identification that’s necessary for effective control, but you may choose to spray an insecticide, either to treat crops that are currently being harvested or as cleanup sprays before terminating a crop. For a list of conventional and OMRI-approved insecticides for aphid control in protected culture, see Table 19: Fungicides and Bactericides Labeled for Vegetable Transplants in the New England Vegetable Management Guide.
Planning Ahead for Successful Aphid Biocontrol
Many biological control agents, especially parasitoids, are only effective on certain aphid species. Correctly identifying the species of aphid affecting your crop is an important first step before selecting which biocontrol organisms will be effective.

- Green peach aphids (Myzus persicae) can vary in color from green to pink to red. They can be distinguished from the melon/cotton aphid by the length and color of the cornicles (the pair of tube-like protrusions extending from the end of the abdomen). Green peach aphids have relatively long cornicles and only the tips are black. In addition, the tubercles (bumps at the base of the antennae) are prominent and turned inwards, appearing as a distinct indentation between the antennae (see photo). Hosts include peach, apricot, and over 200 species herbaceous plants including vegetables and ornamentals.
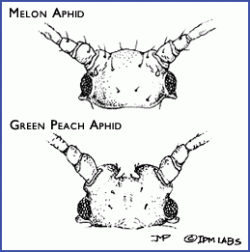
- Melon/cotton aphid (Aphis gossypii): The cornicles on melon/cotton aphid are short and vary in color from light yellow to very dark green (making them appear black). The antennae are typically shorter than the body. Melon/cotton aphids do not have a distinct indentation at the base of the antennae like that of the green peach aphid. Its host range includes hundreds of species, including vegetable crops such as pepper, eggplant, spinach, asparagus, and okra, and it is particularly damaging on cucurbits.
- Foxglove aphid (Aulacorthum solani): Foxglove aphids have dark green flecks at the base of their cornicles. In addition, they have black markings on their leg joints and antennae. Foxglove aphids tend to fall off plants when disturbed. They can cause severe leaf distortion, more so than the green peach and melon/cotton aphid. This aphid has many hosts including foxglove, lettuce, potato, clover, and bulbs.
- Potato aphids (Macrosiphum euphorbiae) can be green or pink and both colors are often found on the same plant. They are larger with a more elongate body shape and move more quickly than most other common aphids.

 These aphids complete 2-6 generations on their winter host of rose plants before moving on to their summer hosts, which include potato and tomato. Therefore, this aphid pest is not typically seen in tunnels until later in the season, but they have been reported as a growing problem among high tunnel tomato growers and keeping an eye out for them early is a good idea.
These aphids complete 2-6 generations on their winter host of rose plants before moving on to their summer hosts, which include potato and tomato. Therefore, this aphid pest is not typically seen in tunnels until later in the season, but they have been reported as a growing problem among high tunnel tomato growers and keeping an eye out for them early is a good idea. - Cabbage aphids (Brevicoryne brassicae) are not typically considered a tunnel pest but have been reported in tunnels with overwintered brassicas. Mature females are greyish green with dark heads and cornicles. Adults produce a powdery wax coating that makes them appear dusty. Cabbage
 aphids are restricted to brassica species.
aphids are restricted to brassica species. - Root aphid: The primary root aphid (Pemphigus species) overwinters as eggs and infests plants in the spring and fall. Root aphids may be misidentified as mealybugs because they are covered with white wax although they are smaller than mealybugs. Root aphids have reduced cornicles that resemble rings, which are located on the end of the abdomen. These cornicles are difficult to see with the naked eye but can be seen when magnified.
Biological Control Using Aphid Predators
In general, aphid predators are better at controlling high aphid populations compared to parasitoids, as they are not as efficient at finding low numbers of aphids within the crop.
- Lady beetles are sold as adults and are effective at quickly controlling high aphid populations, but they are highly dispersive and will readily leave the tunnel in search of food if aphid populations are too low. They can be effective if released under row cover in winter greens. Adults and larvae are generalists, meaning they feed on a wide variety of aphid species.
- Predatory midges (Aphidoletes aphidimyza) are another generalist aphid predator that can be purchased and released in your tunnel. They are active in summer months, but when day lengths shorten to less than 15 hours (September-March), they enter diapause and become inactive. Larvae feed on aphids and adults feed on pollen and aphid honeydew. Banker plants used to support Aphidius colemani (see below) will also support Aphidoletes midges. The midges pupate in the soil, so place banker plants in a tray with moist sand to provide pupation sites if your tunnel has plastic mulch and weed mat.
- Green lacewing larvae also feed on many aphid species. Adults feed on pollen and nectar. Lacewings can be purchased in any life stage; larvae tend to survive better than eggs and are best for immediate pest control. Release far apart from each other, as larvae are cannibalistic. In summer months, if temperatures rise above 95°F, lacewings will move out of the tunnel. Lacewing activity and life cycle slows as temperature drops, but one report from Purdue University reported that adults remained active and laying eggs at 52°F.
In addition to introduced aphid predators, naturally occurring predators such as syrphid hover flies and native lady beetles which make their way into tunnels and greenhouses can also contribute to control of aphid pests. Conservation biological control practices may be used to enhance the populations of these predators in a given area by providing them with resources such as food and shelter. This includes planting insectary plants such as sweet alyssum, which have been shown to attract hover flies whose larvae can provide effective control of aphids.
Biological Control Using Parasitoids
 Aphid parasitoids in the genera Aphidius and Aphelinus are wasps that lay their eggs in the host aphid. The resulting wasp larva develops within the aphid, eating the host from the inside. The remaining shell of the aphid becomes tan or pink and is called a “mummy”. Adult parasitoids emerge from aphid mummies and continue the cycle. Parasitoids are effective for controlling low populations of aphids and preventing outbreaks but are not effective at managing high populations. They are generally more efficient than aphid predators at seeking out the aphid hosts at low levels. Parasitoids are less effective at cold and hot temperatures and function best in the range of 65-77°F and with 70-85% relative humidity. Aphidius does not enter diapause, however, and can be used at colder temperatures.
Aphid parasitoids in the genera Aphidius and Aphelinus are wasps that lay their eggs in the host aphid. The resulting wasp larva develops within the aphid, eating the host from the inside. The remaining shell of the aphid becomes tan or pink and is called a “mummy”. Adult parasitoids emerge from aphid mummies and continue the cycle. Parasitoids are effective for controlling low populations of aphids and preventing outbreaks but are not effective at managing high populations. They are generally more efficient than aphid predators at seeking out the aphid hosts at low levels. Parasitoids are less effective at cold and hot temperatures and function best in the range of 65-77°F and with 70-85% relative humidity. Aphidius does not enter diapause, however, and can be used at colder temperatures.
Aphid parasitoids are host-specific in terms of the aphid species they attack—see the table below for parasitoid-host information. Currently, no parasitoids are commercially available for cabbage and root aphids. Mixtures of different parasitoid species are commercially available and should be used when multiple aphid species are present or when you cannot identify the aphid species in your tunnel. Parasitoids are shipped either as adults or aphid mummies, from which parasitoid adults soon emerge. To increase the parasitoids’ effectiveness, place small groups of the aphid mummies in cups near aphid colonies. Do not let these aphid mummies get wet. Release rates may vary depending on the parasitoid species. Containers often contain approximately 250 aphid mummies, which can treat 5,000 ft2 at the high release rate (for high aphid populations) or 25,000 ft2 at the low release rate (for less severe outbreaks).
Aphid parasitoids must be applied preventatively to suppress aphid populations. They are less effective when aphid populations are high and already causing plant damage. Release parasitoids on a regular basis to sustain their populations during the growing season. Avoid releasing parasitoids near sticky cards to prevent capturing the released parasitoids. When scouting, look for aphid mummies that have circular holes on one end. These are the exit holes created by adult parasitoids during emergence. Aphid parasitoids are sensitive to pesticides. Release parasitoids preventively on crops you know are susceptible to aphids, so that the parasitoids will be present when aphids are first noticed.
Parasitoids are themselves susceptible to parasitism from other wasp species—these wasps that parasitize parasitoids are called hyperparasites. Hyperparasites will move into tunnels throughout the summer and lay eggs within aphids that have already been parasitized by Aphidius species. The hyperparasite larva then feeds on the Aphidius larva and an adult hyperparasite emerges from the aphid mummy. The exit holds of the aphid mummies can be used as an indicator of the hyperparasitoid population; Aphidius wasps leave a round, smooth-edged exit hole while hyperparasitoid wasps leave a slightly irregular exit hole with jagged edges. If you plan on using parasitoids for aphid control year-round in your tunnel, the wasps and/or banker plants will need to be re-introduced once the tunnel has been closed for the winter and the existing hyperparasitoid population has died. For more information on aphid hyperparasites, see the UVM fact sheet Hyperparasitoids of Aphid Predatory Wasps.
|
|
Biocontrol Agent |
Target Species |
Effective Conditions |
Notes |
|---|---|---|---|---|
|
Predators |
Ladybeetles Convergent ladybeetle (Hippodamia convergens) |
All aphid species, in addition to other pests |
Year-round |
Only effective for high populations and if structure is enclosed |
|
Predatory midge (Aphidoletes aphidimyza) |
All aphid species |
Inactive September-March unless supplemental light is provided or temperatures remain above 78°F |
|
|
|
Green lacewing (Chrysoperla rufilabris) |
All aphid species |
Optimal: 60-80°F. Will leave tunnel above 95°F. Lower temp limit unknown but remain active at 50°F. |
Good for high populations |
|
|
Parasitoids |
Aphidius colemani (parasitic wasp) |
Green peach and melon aphids |
65-77°F, 70-85% relative humidity |
Does not enter diapause so is effective during low winter light |
|
Aphidius matricariae (parasitic wasp) |
Green peach and tobacco aphid |
65-77°F, 70-85% relative humidity |
Does not enter diapause so is effective during low winter light. Susceptible to hyperparasitoids in summer. |
|
|
Aphidius ervi (parasitic wasp) |
Foxglove and potato aphid |
65-77°F, 70-85% relative humidity |
Does not enter diapause so is effective during low winter light. Susceptible to hyperparasitoids in summer. |
|
|
Aphelinus abdominalis (parasitic wasp) |
Foxglove and potato aphid |
65-77°F, 70-85% relative humidity |
|
Banker Plant Systems
Banker plant systems are used to maintain parasitoid populations within a tunnel when host pest populations are low, so that the parasitoids do not leave the tunnel looking for hosts. The trade-off of using time and space to grow banker plants and maintain the non-pest aphid population is that you don’t need to continually order and release parasitoids in your tunnels.
In the case of aphids in high tunnel crops, banker plants are used to maintain and distribute populations of Aphidius colemani, which parasitizes green peach and melon aphids. Potted grass plants are inoculated with bird-cherry oat aphids (Rhopalosiphum padi), which feed only on grasses. A. colemani is then released onto the grass plants and parasitize the bird-cherry oat aphids. In this way, the parasitoid population is maintained and the wasps are present in the tunnel when aphids arrive in the main tunnel crop. Recent research from the University of North Carolina found that this system worked best using wheat or barley as the banker crops, compared to oats or rye.
There has been limited research on how many banker plants are needed for a given area, but regardless, banker plants need to be distributed evenly throughout the tunnel, as A. colemani will only migrate 3-6 feet from the point of release/emergence. One rate recommendation given is one banker plant per 1000 ft2. Adjust your banker plant rates based on your experience. As with all parasitoid systems, banker plants need to be in place before the pest aphids are even noticed in order to provide sufficient control. Starter aphid banker plants are available from several biological control suppliers. One starter kit is enough to get your banker plant system started for the season, as long as you’re growing your own pots of oat, rye, or barley.
Entomopathogenic fungus
The entomopathogenic fungus, Beauveria bassiana, is commercially available as the products Mycotrol and BotaniGard. Because aphids have high reproductive rates and molt rapidly, especially during the summer, repeat applications are typically required. Beauveria bassiana is most effective when aphid populations are low. This fungus may not be compatible with the convergent ladybird beetle (Hippodamia convergens) depending on the concentration of spores applied.
Compiled from the following resources:
Aphids on Greenhouse Crops, by Tina Smith, UMass Extension
Managing Aphids in the Greenhouse, Aphid Banker Plants, and Biological Control of Aphids by Leanne Pundt, UConn Extension
Aphid Management in Winter Tunnel Greens, Cornell Cooperative Extension
Other helpful resources:
Aphid Banker Plant System for Greenhouse IPM: Step-by-Step, by Margaret Skinner & Cheryl Frank, UVM Entomology Research Lab and Ronald Valentin, BioBest
Scheduling Biologicals, by Linda Taranto, D&D Farms and Tina Smith, UMass Extension
-- UMass Extension Vegetable Team
News
MDAR Agricultural Education Survey
MDAR is conducting a study on agricultural education as part of its greater workforce development effort.
To help inform this study, please share your insights with our team by completing the survey link. The survey should take less than five minutes, but we encourage longer responses to help inform this effort. Please submit feedback by April 5th.
Thank you for taking the time to share your thoughts with us.
Results: UMass Extension Weeds Survey
Thank you to everyone who participated in the recent UMass Extension weeds survey! These results will help me (Maria Gannett, the new Vegetable and Fruit weeds specialist), figure out the most critical weeds and management techniques to focus on.
Grasses were identified as the most difficult to manage weed. All three of the next most troublesome weeds were perennial weeds: bittersweet, nutsedge, and bindweed. Herbicides and hand weeding were the most commonly used weed management tools. Herbicides were also the tool that people wanted the most information about. I'll post the full survey result summary here shortly.
If you did not get a chance to fill out the survey and you still want to share your thoughts, click here to find the survey.
URI Laser Scarecrows Available
Research at the University of Rhode Island, Cornell Cooperative Extension, and the University of Florida/USDA-APHIS National Wildlife Research Service has shown that the use of automated lasers can significantly reduce bird damage to sweet corn.
The URI Laser Scarecrow Project is making experimental devises available to growers who want to try the technology on their farms. Fully assembled scarecrows are available for $800 each to participating farmers. A single unit has a protection radius of 300-500 ft for sweet corn. Preference will be given to sweet corn growers but the scarecrows can be used in other crops.
For more information or to request a scarecrow, contact Dr. Rebeca Brown at brownreb@uri.edu or 401-874-2755, or visit laserscarecrow.info.
MDAR Grant Programs for Farmers Now Open
Food Security Infrastructure Grant (FSIG)
The FSIG Program seeks to ensure that farmers, commercial fishermen, and other local food producers are better engaged with a strong, resilient food system to help mitigate food supply and distribution disruptions, as well as to ensure that individuals and families throughout the Commonwealth have equitable access to food, with a focus on food that is produced locally. Up to $500,000, on a reimbursement basis.
Applications Due: 4:00 PM on Thursday, May 2, 2024
Contact: Laura Maul at Laura.Maul@mass.gov or (857) 507-5972
Urban Agriculture Program
This program award grants statewide to promote strategies addressing food insecurity, to expand and create new economic opportunities and to increase access to fresh, local produce in urban neighborhoods. $5,000-$50,000 for commercial urban agriculture projects, $1,000-$20,000 for community garden and food production projects, or $150,000 for land acquisition (restrictions apply).
Applications Due: 4:00 PM on Monday, May 6, 2024
Contact: Rose Arruda at Rose.Arrruda@mass.gov or (617) 851-3644
Agricultural Food Safety Improvement Program (AFSIP)
The Agricultural Food Safety Improvement Program (AFSIP) is a reimbursement grant program that supports produce and aquaculture operations that are looking to improve their food safety practices that work towards minimizing the risks of microbial contamination and food-borne illnesses while increasing market access.
Participants selected for funding are provided with reimbursement grants for 80% of total project costs up to $50,000.
Applications Due: 4:00pm on Friday, May 24, 2024.
Contact: Laura Maul at Laura.Maul@mass.gov or 857-507-5972
Climate Smart Agriculture Program (CSAP)
The Climate Smart Agriculture Program links MDAR's water, energy, and climate grants together into one application. This includes the Agricultural Climate Resiliency & Efficiencies (ACRE) Program, the Agricultural Environmental Enhancement Program (AEEP), and the Agricultural Energy Grant Program (ENER). This program continues the goals of the three individual grants by implementing projects that help the agricultural sector adapt to climate change, mitigate climate change, reducing or preventing impacts to natural resources that may result from agricultural practices, and that improve energy efficiency and facilitate adoption of alternative clean energy technologies.
The CSAP grant is broken into two sections.
- Section I: Environment is for environmental projects such as soil health, water use efficiency, or other projects working towards reducing or limiting greenhouse gas emissions.
- Section II: Energy is Ag-Energy projects to improve energy efficiency or to facilitate clean energy adoption.
Applicants can apply to either or both sections.
Participants selected for funding under either section are provided with reimbursement grants for 80% of total project costs up to $50,000.
Applications Due: 4:00pm on Friday, May 31, 2024.
Contacts:
- Section I: Environment - Laura Maul at Laura.Maul@mass.gov or 857-507-5972
- Section II: Energy - Gerry Palano at Gerald.Palano@mass.gov or 617-571-4969
Uses of Chlorpyrifos on Food Products in 2024
In 2021, EPA revoked all tolerances for chlorpyrifos on food crops, meaning it would be unlawful for there to be any residues of it on food and feed, thereby making it illegal to use on food crops at all. However, that ruling was challenged and in November 2023, the 8th Circuit Court of Appeals ruled to vacate EPA’s final rule on chlorpyrifos tolerances and instructed EPA to issue a new rule that takes into consideration several specific uses of this pesticide in agricultural crops that it had described in its initial review of these materials. A new final rule from EPA is pending. EPA has said that it “expects to expeditiously propose a new rule to revoke the tolerances associated with all but the 11 uses referenced by the court…” The uses are limited to 11 crops and specify the regions/states for which they would be considered “high-benefit”. The only vegetable crop identified was asparagus and Massachusetts was not included among the states considered for retention of chlorpyrifos uses during this review.
In the meantime, while we wait for EPA’s new final rule on chlorpyrifos tolerances, the original tolerances for food crops have been reinstated. However, many manufacturers have already voluntarily canceled their registrations or eliminated labeled uses on food crops. A memo issued by USDA to US growers identifies the products that contain chlorpyrifos that currently have registered food uses. Of these 14 products, 3 are only allowed under existing stocks provisions and only 7 are currently registered in Massachusetts. To search the Massachusetts registration status of a pesticide, see https://www.kellysolutions.com/ma/pesticideindex.htm. Current product labels are available at CDMS.
Events
Fundamentals of Organic Production: Organics & Food Safety
When: Wednesday, April 24, 10:00am – 11:30am
Where: Zoom
Registration: Free! Click here to register.
There are points of both overlap and tension for farms trying to meet organic and food safety standards. In this workshop, we’ll cover the principles and requirements of both and the ways that growers can meet them effectively and streamline their efforts as much as possible. Topics will include selecting and handling soil amendments, agricultural water management, and recordkeeping.
This workshop is the last webinar in our Fundamentals of Organic Production series, which is part of the USDA Transition to Organic Partnership Program (TOPP). We do have two upcoming in-person events this summer - June 12 at Tangerini Farm (see event listing below), and a field walk at Astarte Farm on July 9 (details and registration coming soon!). Hope to see you there!
To learn more about the UMass & NOFA/Mass programming, check out the NOFA/Mass TOPP web page. To learn more about the national TOPP program, visit www.organictransition.org.
Recorded Previous Workshops:
Cover Cropping – Part 1 and Part 2
Twilight Meeting at Tangerini's Farm
When: June 12, 2024, 4-7pm
Where: Tangerini's Spring Street Farm,139 Spring Street, Millis, MA, 02054
Registration: Free! Please register in advance, for food ordering purposes. Click here to register.
Join Tangerini Farm, the UMass Extension Vegetable Program and SEMAP for a twilight meeting!
- Steve Chiarizio of Tangerini Farm will describe the new tile drainage system and bioreactor that they installed at the farm last year with the help of Massachusetts FSIG funding.
- Maria Gannett, UMass Extension Weed Specialist, and Sue Scheufele, UMass Extension Vegetable IPM Specialist, will talk about sweet corn weed and insect pest management options.
Presentations 4-6 pm, followed by a light supper.
Program co-sponsored by UMass Extension and the Southeastern Massachusetts Agricultural Partnership (SEMAP)
Pesticide recertification credits TBD
Transition to Organic Partnership Program: Brookfield Farm Field Walk
When: Tuesday, June 25, 2024, 4-7pm
Where: Brookfield Farm, 24 Hulst Rd., Amherst, MA 01002
Registration: Free! Please register in advance, for food ordering purposes. Click here to register.
Join Brookfield Farm, the UMass Extension Vegetable Program and NOFA/Mass for a twilight meeting!
- Kerry and Max Taylor from Brookfield Farm will describe the new well they installed last year that includes a solar pump. The well was installed with support from NRCS.
- Sue Scheufele, UMass Extension Vegetable IPM Specialist, will lead a field walk and pest scouting demonstration.
- Maria Gannett, UMass Extension Weed Specialist, will lead a weed walk and discuss weed ID, organic weed management, and the relationship between weeds and soil health.
Presentations 4-6 pm, followed by a light supper.
Vegetable Notes. Genevieve Higgins, Lisa McKeag, Susan Scheufele, Hannah Whitehead, Alireza Shokoohi, co-editors. All photos in this publication are credited to the UMass Extension Vegetable Program unless otherwise noted.
Where trade names or commercial products are used, no company or product endorsement is implied or intended. Always read the label before using any pesticide. The label is the legal document for product use. Disregard any information in this newsletter if it is in conflict with the label.
The University of Massachusetts Extension is an equal opportunity provider and employer, United States Department of Agriculture cooperating. Contact your local Extension office for information on disability accommodations. Contact the State Center Directors Office if you have concerns related to discrimination, 413-545-4800.










