In This Issue
- Mass Aggie Seminars
- Photo Contest for the 2025 UMass Garden Calendar
- 2024 Perennial Plant of the Year - Phlox paniculata 'Jeana'
- Entomopathogenic Nematodes (EPN) and Their Use as Biocontrol in Managed Landscapes
- Trouble Maker of the Month
- Q&A
- Garden Clippings Tips of the Month
- A Consideration of the Benefits of Using Conifers in the Urban Landscape - Part I
A monthly e-newsletter from UMass Extension for landscapers, arborists, and other Green Industry professionals, including monthly tips for home gardeners.
To read individual sections of the message, click on the section headings below to expand the content.
To print this issue, either press CTRL/CMD + P or right click on the page and choose Print from the pop-up menu.
Mass Aggie Seminars
UMass Amherst officially opened its doors in 1863 as the Massachusetts Agricultural College, and was affectionately known as “Mass Aggie”. UMass Extension honors the tradition of bringing agricultural knowledge to the Commonwealth through this series of seminars - the Mass Aggies - by detailing the best ways to raise fruit in Massachusetts.
Delve into the cutting-edge information shared in our seminars intended for small scale backyard growers and agricultural enthusiasts of all types, and learn about the latest developments on growing fruit. This series highlights the agricultural expertise and innovation available through the University of Massachusetts Amherst’s Extension Fruit Team. Pre-registration required as seating is limited.
Webinars via Zoom
February 17 - Orchard Establishment ($35)
March 2 - Integrated Pest Management & Insect ID ($35)
March 9 - Orchard Pruning ($35)
In-Person at UMass Amherst
February 24 - For the Love of Pollinators ($60)
For more details and to register, go to https://ag.umass.edu/fruit/news-events/mass-aggie-seminars-2024
Photo Contest for the 2025 UMass Garden Calendar
Ever take a great garden photo and think “this would be perfect for the UMass Garden Calendar?” UMass is accepting photos from the public for possible use in the 2025 Garden Calendar.
Photos must be horizontally oriented with high resolution and feature garden plants of particular horticultural interest. Winning photographers will be credited in the Garden Calendar and will receive 5 free calendars. For complete info and submission instructions, go to ag.umass.edu/gardenphotos
2024 Perennial Plant of the Year - Phlox paniculata 'Jeana'
 The Perennial Plant Association has chosen Phlox paniculata ‘Jeana’ as the Perennial Plant of the Year for 2024. 'Jeana' is an exceptional garden phlox renowned for its impressive flower show, tall sturdy habit, and pollinator-friendliness. Dense, domed trusses crown stiff stems from midsummer to early fall. Individually, the fragrant lavender-pink flowers are significantly smaller than typical garden phlox — only about half an inch wide — but the show at peak is eye-popping, nonetheless. This is a case where bigger is not better, from a pollinator’s perspective anyhow. In trials at Mt. Cuba Center in Delaware, the nectar-rich flowers of ‘Jeana’ attracted more butterflies — Eastern Tiger Swallowtails were especially plentiful — than any other garden phlox in their study. Hummingbirds and other pollinators are fans too.
The Perennial Plant Association has chosen Phlox paniculata ‘Jeana’ as the Perennial Plant of the Year for 2024. 'Jeana' is an exceptional garden phlox renowned for its impressive flower show, tall sturdy habit, and pollinator-friendliness. Dense, domed trusses crown stiff stems from midsummer to early fall. Individually, the fragrant lavender-pink flowers are significantly smaller than typical garden phlox — only about half an inch wide — but the show at peak is eye-popping, nonetheless. This is a case where bigger is not better, from a pollinator’s perspective anyhow. In trials at Mt. Cuba Center in Delaware, the nectar-rich flowers of ‘Jeana’ attracted more butterflies — Eastern Tiger Swallowtails were especially plentiful — than any other garden phlox in their study. Hummingbirds and other pollinators are fans too.
Topped with flowers, ‘Jeana’ can reach five feet tall and four feet wide, although size will vary geographically. Its bright green leaves are highly resistant to powdery mildew, so ‘Jeana’ has a striking summer look with or without flowers.
Tall garden phlox provide structure and color in summer gardens and are good bridging plants between early and later flowering perennials. ‘Jeana’ is at home in traditional borders and meadows and is a natural in pollinator gardens. Mix ‘Jeana’ with other tall perennials such as bluestars (Amsonia), Shasta daisies (Leucanthemum ×superbum), and switch grasses (Panicum virgatum). Or let its handsome foliage be the backdrop for shorter companions such as coneflowers (Echinacea), alliums (Allium), and woodland sages (Salvia nemorosa).
For more details, go to https://perennialplant.org/page/2024PPOY.
Entomopathogenic Nematodes (EPN) and Their Use as Biocontrol in Managed Landscapes
Insect management in urban landscapes (turf and ornamentals) has relied on chemical control for decades as the most effective and reliable management strategy. However, negative impacts on the environment, non-target organisms and even human health have raised concerns and exposed the dire need for new alternative approaches. In addition, the development of insecticide resistance by insect populations causes major classes of synthetic insecticides to lose efficacy against insect pests. For instance, golf courses in the northeastern part of the US are facing insecticide resistance problems with annual bluegrass weevils (Listronotus maculicollis). These weevils have developed a resistance to pyrethroids; however, the efficacy of other insecticide classes is affected as a result. Another example is the southern chinchbug. This species has developed resistance to most of the chemical insecticides used to manage them and has even been able to overcome the defenses of resistant grass cultivars. The current concerns regarding safety and efficacy of synthetic insecticides are usually reflected in stricter federal and/or state regulations; for instance, as in the ban of chlorpyrifos, an organophosphate insecticide, in turf, and recent restrictions on neonicotinoid use.
Biological control of landscape insect pests has become an important alternative to synthetic chemicals. The classical definition of biocontrol implies using living organisms to reduce the population of another “pest” species. Using predators, parasitoids and pathogens can be examples of biocontrol implementation. According to recent estimates, the biocontrol market is growing in the US and worldwide. However, it is still far behind the share of the market of conventional insecticides in spite of the growing interest of practitioners and the public to use less toxic management approaches. The global market size of synthetic insecticides is estimated to be around $14 billion, whereas the biopesticide market is around $800 million, and the current market for nematodes is only about $14 million. The market for biological and biorational insecticides is constantly changing. For instance, only few large companies producing nematodes are consistently active over a long period of time and, while many smaller businesses offer these products, they often do not last long. Some of these are listed in Table 1.
Nematodes as a biocontrol agent
Nematodes (round worms) are one of the most numerous and successful multicellular life forms on our planet. They occur in many different habitats; they can be found in the depths of the sea and at high altitudes. The size of different nematode species varies from ~4 mm to ~43 ft. They can be neutral, pest or beneficial in regard to their significance to the ecosystem or human activity and health. Pest nematodes include vertebrate and mammalian parasites and can affect human health (hookworms, threadworms, pinworms, etc.) and domestic animals (heartworms, tapeworms). Plant parasitic nematodes can cause significant economic losses in agriculture and are serious and challenging pests to control.
In contrast, invertebrate parasitizing nematodes can significantly reduce the population of insect pests and other undesirable invertebrates, and therefore are considered as beneficial and are used as biological control agents. Nematodes affecting insects, a.k.a. entomopathogenic nematodes (EPN, entomo = insect), are one of the most effective biocontrol agents/organisms successfully used against insect pests in different crop systems. About 40 families of nematodes are associated with insects, but only two families cause rapid insect mortality (within 48 h... Steinernematidae and Heterorhabditidae) and therefore are suitable as biocontrol.
How nematodes kill their insect host and life cycle
The virulence (or ability to cause significant mortality within the insect population) of Steinernema spp. and Heterorhabdis spp. depends on their ability to carry symbiotic bacteria inside their gut. In this mutualistic relationship, nematodes serve as a carrier or “injection needle” and bacteria are the killing agent. If nematodes have lost their bacteria because of inappropriate storage or shipping but are still alive, these nematodes will not be virulent and will not be able to kill the targeted pest. These bacterial symbionts are beneficial for the nematodes because the bacteria kill the host very fast and thus the nematode can avoid the insect's immune system. Bacteria break down the host tissues into particles that can be digested by the nematodes. In addition, bacteria produce antibiotics, which suppress other pathogens. Nematodes feed on the pre-digested insect tissue and the multiplied bacteria.
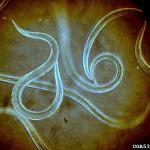 Nematodes can persist in the soil for a relatively long time. It is because they can “freeze” their development at the immature stage, known as infective juveniles (IJs), (Figure 1). Infective juveniles can survive without feeding for a very long time and their sole purpose is to find a host. Their digestive system is not functional and they are not reproductively mature. However, they have a special pouch in their gut where they carry symbiotic bacteria; therefore they are the virulent or infective stage. IJs ability to stay inactive and virulent without additional nutrients for very long periods of time are useful in the formulation and commercialization of the EPNs. Technically, IJs are the “active ingredient” of any formulated nematode product.
Nematodes can persist in the soil for a relatively long time. It is because they can “freeze” their development at the immature stage, known as infective juveniles (IJs), (Figure 1). Infective juveniles can survive without feeding for a very long time and their sole purpose is to find a host. Their digestive system is not functional and they are not reproductively mature. However, they have a special pouch in their gut where they carry symbiotic bacteria; therefore they are the virulent or infective stage. IJs ability to stay inactive and virulent without additional nutrients for very long periods of time are useful in the formulation and commercialization of the EPNs. Technically, IJs are the “active ingredient” of any formulated nematode product.
 Once the infective juveniles find the host, they enter the insect's body, mostly through natural openings (mouth, spiracles, anus). Some Heterorhabditis spp. have a special “tooth” or hook which helps them to break the outer cuticle of the insect and enter the insect body, but penetration through the cuticle is not common and is mostly observed in smaller, soft bodied insects and rarely observed on larger insects. As soon as infective juveniles enter the insect body and start swimming in the insect's blood, they are triggered to resume their development. The symbiotic bacteria are then released into the insect blood (hemocoel) where they multiply, causing septicemia and death of the host insect (within 48h). The nematodes feed on the pre-digested insect tissues and bacteria, finish their development, become reproductivly mature, reproduce, and complete 2-3 generations until the host resources are depleted. Once that happens, nematodes “freeze” in the IJ state (usually 3rd stage) and leave the insect body (Figure 2), later infecting other hosts.
Once the infective juveniles find the host, they enter the insect's body, mostly through natural openings (mouth, spiracles, anus). Some Heterorhabditis spp. have a special “tooth” or hook which helps them to break the outer cuticle of the insect and enter the insect body, but penetration through the cuticle is not common and is mostly observed in smaller, soft bodied insects and rarely observed on larger insects. As soon as infective juveniles enter the insect body and start swimming in the insect's blood, they are triggered to resume their development. The symbiotic bacteria are then released into the insect blood (hemocoel) where they multiply, causing septicemia and death of the host insect (within 48h). The nematodes feed on the pre-digested insect tissues and bacteria, finish their development, become reproductivly mature, reproduce, and complete 2-3 generations until the host resources are depleted. Once that happens, nematodes “freeze” in the IJ state (usually 3rd stage) and leave the insect body (Figure 2), later infecting other hosts.
Nematode host finding behavior
 The host-finding behavior of the infective juveniles varies from species to species. There are three major foraging strategies: cruisers, ambushers, and intermediates. Ambusher species “sit-and-wait” to ambush their hosts. They stand on their tails waving back and forth or bending over in preparation for flinging/attaching themselves to insect hosts passing by, usually surface-dwelling, mobile insects (for example, S. carpocapsae against caterpillars). Cruisers move actively within the soil profile looking for an insect host and are effective against sedentary hosts (for example H. bacteriophora against white grubs, Figure 3). Nematode species with the intermediate host finding strategy utilize both ambushing and cruising (S. feltiae, infects both sedentary and mobile hosts). Choosing the wrong species of nematodes for the targeted pest can significantly decrease the efficacy of a nematode-based insecticide.
The host-finding behavior of the infective juveniles varies from species to species. There are three major foraging strategies: cruisers, ambushers, and intermediates. Ambusher species “sit-and-wait” to ambush their hosts. They stand on their tails waving back and forth or bending over in preparation for flinging/attaching themselves to insect hosts passing by, usually surface-dwelling, mobile insects (for example, S. carpocapsae against caterpillars). Cruisers move actively within the soil profile looking for an insect host and are effective against sedentary hosts (for example H. bacteriophora against white grubs, Figure 3). Nematode species with the intermediate host finding strategy utilize both ambushing and cruising (S. feltiae, infects both sedentary and mobile hosts). Choosing the wrong species of nematodes for the targeted pest can significantly decrease the efficacy of a nematode-based insecticide.
Commercially available nematode species and their use for insect biocontrol
Even though many EPN species naturally occur around the world, only six species have been commercialized and currently available in the US market with relevant stability: four species in the genus Steinernema (S. carpocapsae, S. riobrave, S. feltia, and S. krausei), and two species in the genus Heterorhabditis (H. bacteriophora and H. indica). See Table 1 for nematode species, their possible use, relevant products, and manufacturers.
Nematodes are soil-dwelling organisms, so the soil environment is the most suitable environment for nematode applications. Luckily, more than 90% of insects spend at least some part of their life cycle in the soil (wire worms, white grubs, weevil larvae, fly larvae, crane fly larvae, etc.) or on the soil surface (caterpillars, billbugs). Therefore, soil applications in aqueous solution (spray, irrigation) are the standard nematode application method and have been proven effective for many insect pests.
Because nematodes are UV sensitive and require significant moisture, applications for foliar feeding insects were historically limited. However, with some species foliar application can be effective, especially if care is taken to protect them from UV light (applying on a cloudy day or after sunset). Foliar application found its use in high moisture, protected environments such as greenhouses.
Factors affecting nematode efficacy
Nematodes are living organisms and special care needs to be taken before, during, and after application to ensure their optimal efficacy.
Prior to application. For nematodes to “work” or to be effective, both the nematode IJs and their symbiotic bacteria have to be alive. Therefore, the source of the nematodes, formulation, storage conditions, and duration of storage and shipping can affect nematode survival and virulence (their ability to infect and kill the insect host). Ordering from trusted sources with the shortest delivery option is a key to obtaining a high-quality product. The main recommendation is to ensure the shortest time interval between nematode production and application date. Nematode products usually have a relatively short shelf life and ideally require refrigeration or at least cooling during storage and delivery. If the product was exposed to extreme temperatures even within the expiration date, the quality of such products can be compromised.
A simple “quality test” can be conducted to determine the survival of the IJs if any magnification tool is available; however, the survival of the bacteria and the virulence of the nematodes is impossible to check under field conditions. If quality testing shows high mortality of the IJs in the product, it can indicate that the nematodes were stored or shipped under suboptimal conditions and therefore their virulence can also be affected. Unfortunately, in practice the quality of the received product is rarely checked and if the nematodes do not control the desired pest, it is assumed that they are not an effective tool. Recent observations have demonstrated that the quality of commercially available nematodes has fluctuated drastically among companies, nematode species, and the time of purchase. Some commercial products had 0% of surviving IJs (“active ingredient”) at arrival.
During the application. Nematodes can tolerate high pressure spraying and different nozzle types used during application; however, to reduce the mechanical stress on the IJs, the mesh of the nozzle should be removed before nematode applications. It has been demonstrated that, after soil application, 50% of IJs are lost within hours, mainly because they are inactivated or killed by UV light; after this initial mortality, nematodes die off at the rate of 5-10 % per day. Minimize the amount of initial mortality by making applications early in the morning, at the end of the day, or on cloudy days when the UV intensity is at a minimum. Adding UV protectants in the mix can also improve IJ survival.
The optimal temperature range for the most nematodes is between 70-85ºF, since nematodes become sluggish at lower temperatures (50-60ºF) and are inactivated at high temperatures (85-105ºF). However, there are differences among nematode species; some of the temperature requirements for commercially available nematodes are presented in Table 1.
Many characteristics of the soil can affect survival of nematodes, but moisture is one of the most important factors. IJs use the water film surrounding soil particles or surfaces to move around; therefore, substrate moisture is critical for the survival of the IJs and their host-finding activity. If an application needs to be done during a hot dry season, pre-irrigation can improve efficacy and keeping the area irrigated for about one week after application can improve nematode efficacy.
Table 1. Currently commercially available nematode species, products, and their manufacturers.
Nematode genus |
Nematode species |
Insects targeted |
Foraging strategy |
Temperature requirements |
Product |
Manufacturer |
|---|---|---|---|---|---|---|
| Steinernema |
kraussei | black vine weevil larvae | Nemasys LTM NemaSeekTM |
BASF ArbicoOrganics |
||
| carpocapsae | shore flies, billbugs, caterpillars (cutworms, armyworms, loopers), wireworms/click beetles, flea beetles, craneflies/leatherjackets | ambusher | 70 - 80°F | MilleniumTM Nemasys CTM NemAttackTM CasenemTM Sportnem-TTM (turfgrass) EcomaskTM |
BASF ArbicoOrganics Koppert Biologic |
|
| feltiae | thrips, soil dwelling stages of fungus gnats, leafminers (fly larvae), sycamore lacebugs | intermediate | Infective at 50°F, low temperature nematode. | NemasysTM NemAttckTM NemaShieldTM ScanMaskTM |
BASF ArbicoOrganics Bioworks Biologic |
|
| riobrave | caterpillars, weevil larvae, mole crickets | intermediate | Infective above 95°F, infective range 60-95°F. Infective under dry conditions. | NemAttackTM EntonemTM CapirelTM |
ArbicoOrganics Koppert |
|
| Heterorhabditis |
bacteriophora | white grubs, soil dwelling beetle larvae, weevils larvae (black vine weevil, billbugs) | cruiser | Efficacy drops if temperature <70°F. | Nemasys GTM NemaSeekTM NemashieldHBTM LarvanemTM HeteroMaskTM |
BASF ArbicoOrganics Bioworks Koppert Biologic |
| indica | caterpillars, weevil larvae | Heat tolerant, Infect at 85°F and above. | NemaSeekTM | ArbicoOrganics |
Olga Kostromytska, Stockbridge School of Agriculture, University of Massachusetts Amherst
Trouble Maker of the Month
Cryptocline Needle Blight of Yew (Taxus spp.)
Cryptocline needle blight, caused by the fungal pathogen Cryptocline taxicola, can be a serious disease of ornamental yews in the managed landscape. Based on samples submitted to the UMass Plant Diagnostic Laboratory, English yew (Taxus baccata), Japanese yew (T. cuspidata), and hybrid yew (T. × media) are all susceptible to infection. The pathogen has also been found on Pacific yew (T. brevifolia) and Canada yew (T. canadensis).
Because yews have so few associated diseases, the damage Cryptocline is able to cause can be quite dramatic at times. Cryptocline often attacks the current season's shoots and needles before they mature and harden off. Foliar symptoms can develop as scattered, pale green to brown-colored lesions that expand over time to consume the entire needle. When the shoots die, the needles are typically held in place and become pale brown. Signs of the pathogen are often absent in the field but after brief periods of incubation in moist chambers, dark and rounded fruiting bodies (acervuli) emerge from both the upper (adaxial) and lower (abaxial) surfaces of infected needles. They may develop tufts of gray-colored mycelia that appears similar to Phyllosticta and Botryosphaeria. Discharged spores are splashed or blown onto nearby shoots and needles to initiate new infections. Little is known about the life cycle, but infections most likely develop in the spring and early summer during mild and wet conditions. It's also possible that the fungus can invade tissues in the fall if environmental conditions are ideal. Shade favors the development of most fungal pathogens, as they require moisture on plant surfaces for spores to germinate and infect.
Overall, Cryptocline needle blight does not appear to be a common disease of yews across our managed landscapes. Plants under stress from wet/heavy soils, transplant shock, drought, among other stresses, are more likely to develop significant disease outbreaks. Prune out and discard all blighted shoots from infected plants. If possible, remove or cover the dead, discarded needles at the base of the plant with mulch since they also harbor the pathogen. If left at the site, the fungus will readily overwinter, allowing C. taxicola to sporulate the following spring and potentially infect next year's new growth, perpetuating the disease cycle. Research and trials are lacking for this pathogen and host but broad-spectrum fungicides such as copper-based products and mancozeb should have some utility against the pathogen. Applications should be made in the spring when new growth is half-elongated and regular intervals thereafter if wet conditions persist. Increasing air-flow and sunlight is also recommended to reduce the time that free moisture lingers on shoots and needles.
Photos of Cryptocline needle blight symptoms and signs can be found in this UMass Extension fact sheet at https://ag.umass.edu/landscape/fact-sheets/cryptocline-needle-blight.
Nicholas J. Brazee, UMass Extension Plant Pathologist
Q&A
Q. I would like to scale up my flower borders in a cost-effective fashion. I have started annuals such as zinnias from seed before, but am wondering which perennials would be relatively easy to start from seed?
A. Kudos for considering growing perennials from seed! As you noted at the beginning of your question, it can be a cost-effective way to generate large numbers of plants for filling out beds. Another advantage to growing from seed is the opportunity to grow selections not readily available at the local garden center. A few caveats, however, to seed-starting perennials include the obvious fact that the plants may take longer than a season (or longer than nursery-purchased container plants) to “look like something,” i.e., attain a pleasing size and bloom adequately. It is also possible that several seed-generated plants will not have the desired appearance, although this variability may produce some happy accidents where the resultant plants are more attractive than anticipated.
While there are certainly perennial species (and some annuals, for that matter) that require specific and perhaps challenging germination requirements, a host of others are as straightforward as zinnias to propagate from seed. Often these are plants that are short-lived and that gardeners find reseeding in flowerbeds. But they are not invasive and are readily pulled out or transplanted to another location in the garden. Below are a few of these perennials that fit the profile of easy-from-seed. They need little beyond the basics: a sterile germination medium, light (or not), warmth (around 70°F), and moisture.
- Anise hyssop (Agastache foeniculum): Surface sow or barely cover tiny seeds, which require light to germinate.
- Black-eyed Susan (Rudbeckia spp.): Seeds will germinate without any pre-treatment, but may germinate faster or to a greater percentage if seeds are given a month of cold, moist conditions. (See stratification below.)
- Catmint (Nepeta spp.): Surface sow or barely cover seeds, which require light to germinate.
- Coneflower (Echinacea purpurea): Seeds will germinate without any pre-treatment, but may germinate faster or at a greater percentage if seeds are given one to three months of cold, moist conditions.
- Flax (Linum perenne): Surface sow or barely cover tiny seeds, which require light to germinate. Flax does well when sown directly into garden beds; if they are sown indoors, start seeds in biodegradable pots that can later be planted outdoors to avoid breaking plants’ fragile roots.
- Oriental poppy (Papaver orientale): Surface sow or barely cover tiny seeds, which require light to germinate. Poppies do well when sown directly into garden beds; if they are started indoors, transplant carefully or start in biodegradable pots that can later be planted outdoors to avoid breaking plants’ fragile roots.
- Perennial sunflower (Helianthus spp.): Sow seeds under conditions like those for annual sunflower seeds.
- Pincushion flower/Scabiosa (Lomelosia caucasia): Surface sow or barely cover seeds, which require light to germinate.
- Pinks (Dianthus spp.): Surface sow or barely cover seeds, which require light to germinate.
- Rose campion/Maltese cross (Lychnis coronaria): Surface sow or barely cover seeds, which require light to germinate. Seeds will germinate without any pre-treatment, but may germinate faster or to a greater percentage with two weeks to a month of cold, moist conditions.
- Salvia (Salvia spp.): Surface sow or barely cover seeds, which require light to germinate.
- Yarrow (Achillea millefolium): Surface sow or barely cover seeds.
A wider selection of perennials is available with a little extra effort. To initiate germination, the seeds of these plants may require a period of cold (and/or warmth) known as stratification, nicking or scratching of their hard seedcoat (scarification), or some combination of the two processes. Below are some perennials with an added pre-germination step that still fall into the category of easy germinators.
- Beardtongue (Penstemon spp.): Surface sow seeds. Provide anywhere from three to eight weeks cold, moist stratification depending on species.
- Bee balm (Monarda spp.): Surface sow tiny seeds, and provide cold, moist stratification for one to three months for improved germination. Some bee balm species will germinate without a cold treatment.
- Blazing star (Liatris spp.): Barely cover seeds. Provide two months of cold, moist stratification.
- Columbine (Aquilegia spp.): Surface sow seeds, and cold, moist stratify for three weeks. Some columbine species will germinate without a cold treatment.
- Lupine (Lupinus spp.): Scarify seeds prior to sowing. Start in biodegradable pots that can later be planted outdoors to avoid disturbing plants’ roots.
- Ornamental onion (Allium spp.): Surface sow or barely cover seeds. Depending on the species, Alliums may require anywhere from no cold treatment up to two months of cold, moist stratification.
- Rose mallow (Hibiscus lavatera, H. moscheutos): Scarify seeds prior to sowing. Cold, moist stratify for two months.
- Tickseed (Coreopsis spp.): Surface sow or barely cover seeds, which require light for germination. Provide up to two months of cold, moist stratification, although some species may germinate without treatment.
References:
Cullina, W. 2000. Growing and Propagating Wildflowers. Houghton Mifflin Co., Boston, MA. 322 pp.
Ondra, N. J. 2009. The Perennial Care Manual. Storey Publishing, North Adams, MA. 375 pp.
Jennifer Kujawski, Horticulturist
Garden Clippings Tips of the Month
February is the month to . . . .
-
While browsing through seed catalogs, look for tomato cultivars with disease resistance. Tomato disease codes are V=Verticillium, F=Fusarium, T=Tobacco Mosaic Virus. Use a heat mat to speed up germination of seeds.
-
Start leeks, onions, and celery indoors now. Use soilless mix for germinating seeds indoors.
-
Plant a container herb garden on a sunny kitchen windowsill.
-
Recognize that deer ticks can be active any time the temperature is above freezing, so even at this time of year be vigilant for deer ticks while working outdoors. Take preventative measures to reduce the chance of ticks attaching such as frequent tick checks; wearing light-colored, protective clothing; and treating clothes with permethrin (following label instructions).
-
Clean and repair garden tools. Paint handles of easily-misplaced hand tools a bright color.
-
Prune while trees and shrubs are still fully dormant and while you can better see the branch structure before spring leaf-out. In particular, this is a good time to prune fruit trees, blueberry bushes, and grapes before new growth begins.
-
Scout preferred plants for any evidence of deer or rabbit browse. Reapply deer repellents at temperatures above 32°F to avoid contact freeze injury.
-
Replant frost-heaved plants when soil is not frozen or covered with mulch.
-
Resolve to be a "messy" gardener this spring and try and leave fallen leaves and dead stems in place until the end of May if possible. This gives the larvae of many native bees and other beneficials that overwinter in this debris time to mature and complete their life cycle.
-
Remember that winter salt spray can damage plants as far away as 30 feet. Note where salt-laden snow melt collects and monitor these areas for salt injury in spring.
-
Order tree seedlings from your local Conservation District. Find the one nearest you at https://massacd.org/conservationdistricts.
-
Keep an eye out for spotted lanternfly, even in the winter. For more information about this invasive insect, photos, and currently known locations in Massachusetts, go to https://ag.umass.edu/landscape/fact-sheets/spotted-lanternfly
UMass Extension Landscape, Nursery and Urban Forestry Program staff
A Consideration of the Benefits of Using Conifers in the Urban Landscape - Part I
Urban foresters and arborists working in New England have much to consider when it comes to deciding about the tree species that should be planted along community streets, greenways, parks and landscapes. From wintertime shading to species diversity, and a myriad of considerations in-between, our urban forests require thought and attention to be maintained in a healthy manner and thus provide us with invaluable ecosystem services. Many of these services are, after all, vitally important and include carbon sequestration, wildlife habitat, privacy screening, and storm water abatement through the interception of rainfall. In this two-part article, we will explore the uses and benefits associated with the installation of evergreen conifers in the urban landscape.
 Year-round leaf cover, intuitively, has been shown to provide year-round ecosystem services. The vast majority of coniferous trees in the Northeast are evergreen, meaning many of the aforementioned ecosystem services are offered on a 12-month basis. Many of the benefits derived from deciduous trees, such as pollution absorption, privacy screening, thermal buffering, and rainwater interception, decrease to negligible levels during the leaf-off season. The foliage of evergreen coniferous trees, however, continues to offer these benefits throughout the entire year. Notably, conifers continue to intercept (see Fig. 1) and store rainwater, helping to reduce runoff at a time when soils may be frozen and surface water movement may be particularly acute. Studies show that evergreen trees can intercept and store nearly 50 percent more storm water annually than comparably-sized deciduous trees. Their evergreen foliage also continually absorbs important urban pollutants like ozone, carbon dioxide, and particulate matter (microscopic dust) during drought conditions and during the winter season – two important periods when ozone is being emitted due to the increased use of energy to cool and warm buildings. In addition to these important ecosystem services being derived year-round, evergreen conifers may add some diversity to the built environment.
Year-round leaf cover, intuitively, has been shown to provide year-round ecosystem services. The vast majority of coniferous trees in the Northeast are evergreen, meaning many of the aforementioned ecosystem services are offered on a 12-month basis. Many of the benefits derived from deciduous trees, such as pollution absorption, privacy screening, thermal buffering, and rainwater interception, decrease to negligible levels during the leaf-off season. The foliage of evergreen coniferous trees, however, continues to offer these benefits throughout the entire year. Notably, conifers continue to intercept (see Fig. 1) and store rainwater, helping to reduce runoff at a time when soils may be frozen and surface water movement may be particularly acute. Studies show that evergreen trees can intercept and store nearly 50 percent more storm water annually than comparably-sized deciduous trees. Their evergreen foliage also continually absorbs important urban pollutants like ozone, carbon dioxide, and particulate matter (microscopic dust) during drought conditions and during the winter season – two important periods when ozone is being emitted due to the increased use of energy to cool and warm buildings. In addition to these important ecosystem services being derived year-round, evergreen conifers may add some diversity to the built environment.
With upwards of 50% of our street trees in New England being comprised of maples (Acer spp.), it is obvious that our northeastern urban forests demonstrate a uniformity that is notoriously unsustainable. Urban planting recommendations pertaining to species diversification vary and include:
- No more than 10% of any one species in an urban forest;
- No more than 5% any one species and 10% of any one genus;
- No more than 10% of any one species, 20% of any one genus, and 30% of any one family in the urban forest.
 By simply planting conifers in our urban landscapes, we can help to address this diversity deficit and “spread the risks” related to insects and diseases that threaten to devastate our urban tree populations. We have seen firsthand, after all, the large numbers of urban tree populations that have been lost as a result of insects and diseases that have included chestnut blight (Cryphonectria parasitica), Dutch elm disease (Ophiostoma ulmi), Asian longhorned beetle (Anaplophora glabripennis), and now emerald ash borer (Agrilus glabripennis). The more diverse the tree species composition of an urban forest (see Fig. 2), the more resilient it may be in the face of a disturbance like a pest invasion.
By simply planting conifers in our urban landscapes, we can help to address this diversity deficit and “spread the risks” related to insects and diseases that threaten to devastate our urban tree populations. We have seen firsthand, after all, the large numbers of urban tree populations that have been lost as a result of insects and diseases that have included chestnut blight (Cryphonectria parasitica), Dutch elm disease (Ophiostoma ulmi), Asian longhorned beetle (Anaplophora glabripennis), and now emerald ash borer (Agrilus glabripennis). The more diverse the tree species composition of an urban forest (see Fig. 2), the more resilient it may be in the face of a disturbance like a pest invasion.
The need for diversification, however, doesn't just stop at the tree species level. Studies have shown that diversity at several different hierarchical levels across the urban landscape – including spatial distribution, plant functional type, structure, and age class – is of great benefit to the urban ecosystem. These factors all intermingle to help to create a complex urban forest system that supports efficiency and functionality. By adding evergreen conifers to the core fabric of the urban ecosystem, diversity is increased at each of these important levels, thereby lowering the risk of any single disturbance (e.g., snow/ice storm, drought, or the aforementioned pest outbreak) from devastating large portions of the urban forest by limiting the percentage of trees that could potentially be affected.
 Another benefit derived from trees with dense, evergreen foliage is the associated wildlife habitat, thermal cover, and food resources that are made available during the winter. This helps to increase the presence of urban wildlife since there is often a dearth of suitable wildlife resources in the urban environment. Several species of birds that overwinter in New England use the thermal cover afforded by conifers to take shelter during harsh weather. Birds, squirrels and other small mammals use the cones as food (see Fig. 3), and many of these same species use the thick foliage for cover during the breeding season.
Another benefit derived from trees with dense, evergreen foliage is the associated wildlife habitat, thermal cover, and food resources that are made available during the winter. This helps to increase the presence of urban wildlife since there is often a dearth of suitable wildlife resources in the urban environment. Several species of birds that overwinter in New England use the thermal cover afforded by conifers to take shelter during harsh weather. Birds, squirrels and other small mammals use the cones as food (see Fig. 3), and many of these same species use the thick foliage for cover during the breeding season.
Though their benefits are numerous, it is important to note that evergreen conifers do come with their own suite of insect and disease pests, as well as other concerns regarding their possible disservices, such as winter shading. Though these concerns should not preclude the use of evergreen conifers in urban areas, these are but a few of the distinctions that will need to be accounted for as communities and individuals consider the installation of more evergreen trees. Indeed, as with any type of tree, the positive attributes associated with their installation should be weighed against the negative ones to ensure that a properly informed planting selection has been made. By taking into account ‘Right Tree, Right Place,’ as well as the site objectives (e.g., rainwater interception, species diversity, microclimate management, etc.), the urban forester can indeed create a dynamic and diverse infrastructure system: the urban forest of the 21st century that includes perhaps a multitude of both deciduous and evergreen coniferous species. In Part II next month, we will discuss the application and techniques associated with utilizing conifers in the urban landscape.
J. Casey Clapp and Richard W. Harper, Ph.D., Extension Associate Professor of Urban & Community Forestry, Dept of Environmental Conservation, University of Massachusetts Amherst
Upcoming Events
For details and registration options for these upcoming events, go to the UMass Extension Landscape, Nursery, and Urban Forestry Program Upcoming Events Page.
-
Feb 27 - Community Tree Conference, live via Zoom, 8:00 am to 4:00 pm. Credits available: 2 pesticide contact hours for categories 29, 35, 36, and Applicators License. Association credits: 6.75 ISA, 6.5 (cat 1) SAF, 5.75 (cat 1) and 1 (cat 2) CFE, 2 MCA, 2 MCLP, and 1 MCH.
-
Mass Aggie Seminars - For more details and to register, go to https://ag.umass.edu/fruit/news-events/mass-aggie-seminars-2024
- February 17 - Orchard Establishment ($35), live webinar via Zoom
- February 24 - For the Love of Pollinators ($60), in-person at UMass Amherst
- March 2 - Integrated Pest Management & Insect ID ($35), live webinar via Zoom
- March 9 - Orchard Pruning ($35), live webinar via Zoom
Additional Resources
For detailed reports on growing conditions and pest activity – Check out the Landscape Message
For professional turf managers - Check out our Turf Management Updates
For commercial growers of greenhouse crops and flowers - Check out the New England Greenhouse Update website
For home gardeners and garden retailers - Check out our home lawn and garden resources
TickTalk webinars - To view recordings of past webinars in this series, go to: https://ag.umass.edu/landscape/education-events/ticktalk-with-tickreport-webinars
Diagnostic Services
Landscape and Turf Problem Diagnostics - The UMass Plant Diagnostic Lab is accepting plant disease, insect pest and invasive plant/weed samples. By mail is preferred, but clients who would like to hand-deliver samples may do so by leaving them in the bin marked "Diagnostic Lab Samples" near the back door of French Hall. The lab serves commercial landscape contractors, turf managers, arborists, nurseries and other green industry professionals. It provides woody plant and turf disease analysis, woody plant and turf insect identification, turfgrass identification, weed identification, and offers a report of pest management strategies that are research based, economically sound and environmentally appropriate for the situation. Accurate diagnosis for a turf or landscape problem can often eliminate or reduce the need for pesticide use. See our website for instructions on sample submission and for a sample submission form at http://ag.umass.edu/diagnostics.
Soil and Plant Nutrient Testing - The lab is accepting orders for Routine Soil Analysis (including optional Organic Matter, Soluble Salts, and Nitrate testing), Particle Size Analysis, Pre-Sidedress Nitrate (PSNT), Total Sorbed Metals, and Soilless Media (no other types of soil analyses available at this time). Testing services are available to all. The lab provides test results and recommendations that lead to the wise and economical use of soils and soil amendments. For updates and order forms, visit the UMass Soil and Plant Nutrient Testing Laboratory web site.
Tick Testing - The UMass Center for Agriculture, Food, and the Environment provides a list of potential tick identification and testing options at: https://ag.umass.edu/resources/tick-testing-resources.
