UMass Extension's Landscape Message is an educational newsletter intended to inform and guide Massachusetts Green Industry professionals in the management of our collective landscape. Detailed reports from scouts and Extension specialists on growing conditions, pest activity, and cultural practices for the management of woody ornamentals, trees, and turf are regular features. The following issue has been updated to provide timely management information and the latest regional news and environmental data.
The Landscape Message will be updated bi-monthly in August. The next message will be posted on August 20. To receive immediate notification when the next Landscape Message update is posted, be sure to join our e-mail list
To read individual sections of the message, click on the section headings below to expand the content:
Scouting Information by Region
Environmental Data
The following data was collected on or about August 4, 2021. Total accumulated growing degree days (GDD) represent the heating units above a 50° F baseline temperature collected via regional NEWA stations for the 2021 calendar year. This information is intended for use as a guide for monitoring the developmental stages of pests in your location and planning management strategies accordingly.
|
MA Region/Location |
GDD |
Soil Temp |
Precipitation |
Time/Date of Readings |
||
|
2-Week Gain |
2021 Total |
Sun |
Shade |
|||
|
CAPE |
276 |
1569.5 |
69 |
65 |
0.21 |
12:00 PM 8/4 |
|
SOUTHEAST |
262 |
1627 |
73 |
68 |
0.50 |
5:30 PM 8/4 |
|
NORTH SHORE |
271.5 |
1704 |
67 |
63 |
1.16 |
10:30 AM 8/4 |
|
EAST |
278.5 |
1723.5 |
72 |
68 |
0.93 |
5:00 PM 8/4 |
|
METRO |
251.5 |
1614 |
64 |
63 |
1.00 |
6:15 AM 8/4 |
|
CENTRAL |
260.5 |
1666.5 |
68 |
64 |
1.80 |
7:00 AM 8/4 |
|
PIONEER VALLEY |
260.5 |
1685.5 |
69 |
65 |
0.95 |
11:00 AM 8/4 |
|
BERKSHIRES |
214 |
1384 |
69 |
61 |
3.48 |
7:00 AM 8/4 |
|
AVERAGE |
259 |
1622 |
69 |
65 |
1.25 |
_ |
|
n/a = information not available |
||||||
As of 8/3, there is a "moderate drought" status for the mid Cape and Nantucket and an "abnormally dry" status for the upper and lower Cape: https://droughtmonitor.unl.edu/CurrentMap/StateDroughtMonitor.aspx?MA
Municipal water restrictions map dated 7/29: https://www.mass.gov/doc/water-use-restrictions-map/download
Phenology
| Indicator Plants - Stages of Flowering (BEGIN, BEGIN/FULL, FULL, FULL/END, END) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PLANT NAME (Botanic / Common) | CAPE | S.E. | N.S. | EAST | METRO W. | CENT. | P.V. | BERK. |
|
Polygonum cuspidatum (Japanese knotweed) |
* |
Begin |
* |
* |
* |
* |
* |
* |
|
Clethra alnifolia (summersweet clethra) |
Begin/Full |
Full |
Begin/Full |
Full |
Full |
Full |
Full |
* |
|
Hydrangea paniculata (panicle hydrangea) |
Full |
Begin/Full |
Begin/Full |
Full |
Full |
Full |
Begin/Full |
Begin/Full |
|
Hibiscus syriacus (rose-of-Sharon) |
Full |
Full/End |
Full |
Full |
Full |
Full/End |
Full/End |
Full |
|
Lythrum salicaria (purple loosestrife) |
Full |
Full/End |
Full |
Full |
Full |
Full |
Full/End |
Full |
|
Campsis radicans (trumpet vine) |
Full/End |
End |
End |
Full |
Full |
Full |
Full/End |
Full |
|
Oxydendrum arboreum (sourwood) |
Full/End |
Full/End |
Full/End |
Full |
Full/End |
Full/End |
Full/End |
* |
|
Buddleia davidii (butterfly bush) |
Full/End |
End |
Full/End |
Full |
Full/End |
Full/End |
Full/End |
Full/End |
| * = no activity to report/information not available | ||||||||
Regional Notes
Cape Cod Region (Barnstable)
General Conditions: The average temperature for the period from July 21 thru Aug. 4 was 70˚F with a high of 88˚F on July 27 and a low of 53˚F on August 1. The period has been dominated by mostly sunny days with highs in the 70s and lows in the 60s. Less than a quarter inch of precipitation fell during the two-week period, occurring on July 30 and August 2. Soil moisture is short and the US drought monitor lists portions of the Cape abnormally dry and portions in moderate drought. Woody plants seen in bloom the past two weeks include sourwood, rose of Sharon, chaste tree, mimosa, summersweet, butterfly bush, as well as oak, panicle and bigleaf hydrangeas. Herbaceous plants seen in bloom during the period include daylily, liatris, echinacea, shasta daisy, coreopsis, lavender, hosta, Salvia yangii (previously known as Perovskia atriplicifolia, commonly called Russian sage), creeping bellflower, achillea, butterfly weed, Baptisia tinctoria, veronicastrum, blue vervain, beebalm, garden phlox, black-eyed Susan, balloon flower, and cutleaf coneflower.
Pests/Problems: Insect pests or damage seen include Japanese beetle on various plants, daylily leafminer damage on daylily, euonymus scale on euonymus, white prunicola scale on lilac, scale on tupelo, black turpentine beetle on pitch pine, pine tip moth on pitch pine (causing significant damage on young trees across mid and outer Cape), hibiscus sawfly on hardy hibiscus, thrips on gladiolus and crocosmia, azalea lacebug on azalea, andromeda lacebug on andromeda, oak shothole leafminer damage on oak, eriophyid mites on black cherry, tupelo, and birch. After several seasons of high lecanium scale populations, it is now difficult to find in the areas I have scouted. Plant disease symptoms or signs observed during the period include maple anthracnose, sycamore anthracnose, both powdery and downy mildews and black spot on rose, leaf spot on river birch, apple scab and cedar-apple rust on crabapple, aster yellows on purple coneflower, cercospora leaf spot on hydrangea, powdery mildew on smokebush, dogwood, and numerous perennials, anthracnose on dogwood, guignardia leaf blotch on horsechestnut, and needlecast on pitch pine. Physiological leaf scorch is showing up on dogwoods due to low soil moisture. Weeds and wildflowers seen in bloom were sheep’s bit (Jasione montana), spotted knapweed, rabbitfoot clover, chicory, carpetweed, prostrate spurge, Pennsylvania smartweed, annual fleabane, pokeweed, white clover, birdsfoot trefoil, yellow toadflax, Queen Annes’ lace, goldenrod, pepperweed, horseweed, tansy, pilewort, cat’s ear, and narrowleaf plantain. Don’t forget to protect yourself from mosquitoes and ticks. Rabbits continue to be a landscape problem for most people.
Southeast Region (Dighton)
General Conditions: It's been hard to find fault with the weather over the past two weeks, presented with mild days and frequent light showers in the evening. Remarkably, even unirrigated lawns are still green and show few signs of stress. In a more typical summer most lawns would already be drought dormant. Among the many plants in flower, I've noticed these: Acalypha virginica (Virginia copperleaf), Albizia julibrissin (mimosa), Alcea rosea (hollyhock), Ambrosia artemisiifolia (annual ragweed), Asclepias incarnata (swamp milkweed), Vernonia noveboracensis (New York ironweed), Buddleia davidii (butterfly bush), Campanula rapunculoides (creeping bellflower), Campsis radicans (trumpet vine), Centaurea stoebe (spotted knapweed), Cirsium arvense (Canada thistle), Clethra alnifolia (summersweet), Daucus carota (Queen Anne's lace), Echinacea purpurea (purple coneflower), Eutrochium purpureum (Joe-pye-weed), Hemerocallis (daylily), Hibiscus syriacus (rose of Sharon), Hosta spp. (plantain lily), Hydrangea macrophylla (bigleaf hydrangea), H. paniculata (panicled hydrangea), Hypericum perforatum (St. John's wort), Impatiens capensis (jewel weed), Iliamna rivularis (streambank wild hollyhock), Liatris spicata (blazing star), Lilium lancifolium (tiger lily), L. orientalis (Oriental lily), L. superbum (Turk's cap lily), Linaria vulgaris (toadflax), Lotus corniculatus (bird’s-foot trefoil), Lysimachia clethroides (gooseneck loosestrife), Monarda spp. (beebalm), Nuphar lutea (spadderdock, yellow water lily), Nymphaea odorata (American white water lily), Oenothera biennis (evening primrose), Oxydendrum arboreum (sourwood), Phlox paniculata (garden phlox), Hieracium pilosella (yellow hawkweed), Pontederia cordata (pickerel weed), Rosa 'Knockout', Rudbeckia hirta (black-eyed-Susan), Salvia yangii (Russian sage), Sedum 'Autumn Joy', Solidago spp. (goldenrod), Spiraea alba (meadowsweet), Tanacetum vulgare (Tansy), and Vitex agnus-castus (chaste tree).
Pests/Problems: Crabgrass now has several tillers. The following invasive plants are in flower: Lythrum salicaria (purple loosestrife), Japanese knotweed (Reynoutria japonica, syn. Fallopia japonica, Polygonum cuspidatum), Phragmites australis (common reed), Artemisia vulgaris (mugwort), Cyperus esculentus (yellow nutsedge), Calystegia sepium (hedge bindweed), and Erigeron canadensis (horseweed).
North Shore (Beverly)
General Conditions: This two-week period was mild with scattered rain showers on some days. Approximately 1.16 inches of rain were recorded at Long Hill during this period. Air temperature ranged from low 50s to mid 80s. The average daily temperature was 69℉. The average daily maximum temperature was 78°F and the daily minimum temperature was 61℉. The maximum temperature of 88℉ was recorded on July 26 and the minimum temperature of 56℉ was recorded on August 2. Woody plants seen in bloom include: bottlebrush buckeye (Aesculus parviflora), the bee-bee tree or Korean evodia (Tetradium daniellii), sourwood (Oxydendrum arboreum), silk tree or mimosa (Albizia julibrissin), panicle hydrangea (Hydrangea paniculata), summersweet clethra (Clethra alnifolia), Chinese chastetree (Vitex chinensis), chaste tree (Vitex agnus-castus), butterfly bush (Buddleia davidii), rose-of-Sharon (Hibiscus syriacus), harlequin glorybower (Clerodendrum trichotomum), mountain stewartia (Stewartia ovata), and Castor-aralia (Kalopanax pictus). Herbaceous plants seen in bloom include: milkweed (Asclepias spp.), globe thistle (Echinops ritro), garden phlox (Phlox paniculata), purple coneflower (Echinacea purpurea), black-eyed Susan (Rudbeckia spp.), hostas (Hosta spp.), bush cinquefoil (Potentilla fruticans), cranesbill (Geranium spp.), sedums (Sedum spp.), prairie gay feather (Liatris spicata), yarrow (Achillea millefolium), fairy candles/black cohosh/black bugbane/black snakeroot (Actaea racemosa), yellow corydalis (Corydalis lutea), summer flowering roses (Rosa spp.), Clematis vines (Clematis paniculata), American white water lily (Nymphaea odorata) and an assortment of annuals.
Pests/Problems:

 High pH induced iron deficiency symptoms (interveinal chlorosis) were observed on blueberry plants and azaleas. Powdery mildew was observed on garden phlox and susceptible lilacs. Japanese beetles were also observed causing problems on some plants. Weeds are thriving due to moist soil and warm temperatures. Oriental bittersweet (Celastrus orbiculatus) is growing vigorously. Oriental bittersweet seedlings are easy to remove when the soil is moist and the population is small. Pull steadily and slowly to minimize soil disturbance. Remember that ticks and mosquitoes are still very active. Protect yourself with insect repellent when working outdoors especially at dawn and dusk.
High pH induced iron deficiency symptoms (interveinal chlorosis) were observed on blueberry plants and azaleas. Powdery mildew was observed on garden phlox and susceptible lilacs. Japanese beetles were also observed causing problems on some plants. Weeds are thriving due to moist soil and warm temperatures. Oriental bittersweet (Celastrus orbiculatus) is growing vigorously. Oriental bittersweet seedlings are easy to remove when the soil is moist and the population is small. Pull steadily and slowly to minimize soil disturbance. Remember that ticks and mosquitoes are still very active. Protect yourself with insect repellent when working outdoors especially at dawn and dusk.
East Region (Boston)
General Conditions: July temperatures ranged from a high of 92˚F to several daytime highs in the 70’s. We received an unprecedented 10.47 inches of precipitation for the month of July. August began with three consecutive days of no precipitation. The landscape is lush with many perennials in bloom. An abundance of hummingbird activity has been observed on red buckeye (Aesculus pavia) flowers.
Pests/Problems: Fungal pathogens continue to thrive in the moist conditions. A variety of leaf spot is visible on the foliage of most plants. Outbreaks of fireblight have been observed on Malus spp. (crabapples). Lacebug damage is visible on the foliage of Pieris japonica (Japanese andromeda). Slugs continue to ravage hosta, zinnia and vegetable gardens. The large seed pod masses of Ailanthus altissima (tree of heaven) are easily seen on these invasive trees. Phytolacca americana (pokeweed) purple fruit is maturing. The orange and yellow parasitic tendrils of Cuscuta spp. (dodder) is flowering.
Metro West (Acton)
General Conditions: The weather for the month of July has been all about the rain and it will certainly be spoken about for years to come! The historical monthly average rainfall for the month of July is 4.07” and a total of 9.4” of rain was recorded for this July, with some amount of precipitation including a trace of rain recorded every day this month except for three days, July 15th, 20th, and 31st. Observed in some stage of bloom these past two weeks were the following woody plants: Albizia julibrissin (silk tree), Buddleia spp. (butterfly-bush), Clethra alnifolia (summersweet), Hibiscus syriacus (rose-of-Sharon), Hydrangea paniculata (panicle hydrangea) and its many cultivars including 'Tardiva', Oxydendron arboreum (sourwood), Potentilla fruiticosa (potentilla), Rosa spp. (rose), R. rugosa (Asian beach rose), and R. 'Knockout' (knockout family of roses). Woody vines observed in bloom include Campsis radicans (trumpet vine) and Clematis spp. (clematis). Contributing even more color and interest to the landscape are some flowering herbaceous plants including: Actaea matsumurae 'White Pearl' (bugbane), Alcea rosea (hollyhock), Achillea millefolium (yarrow), Asclepias syriaca (common milkweed), A. tuberosa (butterfly weed), Astilbe spp. (false spirea), Boltonia asteroides (Bolton’s aster),Campanula takesimana ‘Elizabeth’ (bell flower), Cichorium intybus (chicory), Coreopsis sp. (tickseed), C. verticillata (threadleaf coreopsis), Daucus carota (Queen Anne's lace), Echinacea purpurea (purple coneflower) and its many cultivars, Eutrochium purpureum (Joe Pye weed), Geranium spp. (cranesbill), Hemerocallis fulva (orange daylily), H. 'Stella D'Oro', (daylily), H. spp. (daylily), Hosta spp. (plantain lily), Kirengeshoma palmata (yellow wax bells), Lamium maculatum (dead nettle), Leucanthemum sp. (shasta daisy), Liatris spicata (spike gayfeather), Lilium spp. (lily), Lobelia cardinalis (cardinal flower), Lysimachia clethroides (gooseneck loosestrife), Lythrum salicaria (loosestrife), Monarda didyma (scarlet beebalm), Nepeta spp. (ornamental catmint), Patrinia gibbosa (patrinia), Salvia yangii, previously known as Perovskia atriplicifolia (Russian sage), Phlox carolina (Carolina phlox), P. paniculata (garden phlox), Physostegia virginiana (obedient plant), Platycodon grandiflorus (balloon flower), Rudbeckia fulgida var. sullivantii 'Goldsturm' (black eyed Susan), Rudbeckia hirta (black eyed Susan), Senna marilandica (wild senna), Solidago spp. (goldenrod), and Veronicastrum virginicum (Culver’s root).
Pests/Problems: Observed in the landscape these past two weeks were leaf blotch on Aesculus sp. (horsechestnut) and cedar apple rust on Amelanchier spp. (serviceberry) and Crataegus spp. (hawthorn).
Central Region (Boylston)
General Conditions: The second half of July was drier than the first half, but still quite wet for mid-summer. We had seasonably warm temperatures, with daily high’s in the 80’s and low 90’s for the first week, followed by a return to cooler temperatures to finish out the month. Over the past few years, most of us have grown accustomed to extended drought periods in summer. This year, July brought more than 12 inches of rain. While it may sound like a blessing to have so much precipitation at the height of summer, it has made gardening exceptionally difficult. New plantings, especially in poorly drained soils have succumbed to root rot just weeks after planting. That being said, the landscape appears lush and beautiful at a time of year when it can often look scorched. Goldenrods are beginning to show some color across the region. Panicle hydrangeas appear to be at their peak, and we’re already seeing evidence of some early fall color.
Pests/Problems: Minor problems in the landscape at this time of year. Waterlogged soils have made some gardening activities difficult, although watering needs for the most part have been light. Powdery mildew was observed on Monarda fistulosa (wild bergamot). Viburnum leaf beetle damage was observed on Viburnum dentatum (arrowwood viburnum). Slug and snail activity remains high. Mosquito populations have exploded across the region thanks to all the wet weather.
Pioneer Valley Region (Amherst)
General Conditions: Late summer has descended on the Pioneer Valley as we settle into August. This time of year, thick morning fog can blanket the Connecticut River, giving the morning sun a hazy appearance. At night, there’s been a steady chorus of mating crickets and katydids. After the final wave of heavy rainfall on 7/19–20, we have enjoyed a fairly dry and sunny stretch of weather. Although scattered showers and strong thunderstorms did occur on 7/25, 7/27, 7/29 and 8/1, accumulations were dramatically lower compared to earlier in the month, allowing saturated soils a chance to drain. Final accumulation for the month of July at the Easthampton gauge totaled 10.94”, while the South Deerfield gauge tallied 10.81”. Surely, a month for the record books and a nice summary of the record wet July can be found here. The drier conditions and high pressure from the north has also meant cooler temperatures and comfortable dew points. After weeks of swampy humidity levels, the latter is most welcome. It was nearly autumn-like as July passed into August, with high temperatures cresting in the 70s, moderate winds, and lows dipping to the lower 50s. Lawns are green and still growing fast and mature trees and shrubs received the deep, mid-summer watering they needed after the drought and blistering heat of 2020. While the abundance of rain has created its own set of problems, the benefits prevail and hopefully we can ride out the growing season without any prolonged periods of dry and hot weather. For stressed trees and shrubs and those in wet locations, there’s been a noticeable reddening of the foliage. Anthocyanins, which are responsible for the brilliant red color in some deciduous trees (e.g. red maple, tupelo, burning bush) are produced to avoid scorch while chlorophyll production diminishes late in the growing season. However, presently it appears as a dull red and may be in response to bright sun after weeks of cloudy conditions. Again, this is appearing on trees and shrubs in flooded settings (i.e. red maple swamps) and those that are stressed and weakened. The persistent rainfall fueled a surge in mosquito and midge populations. It’s almost impossible to be outside at dawn and dusk without some form of mosquito repellent. While for many it’s been a while since the irrigation system was turned on or the hose was dragged around, it’s time to start thinking about resuming irrigation for certain plants. While it was nice not having to irrigate for most of July, recently transplanted trees and shrubs and those with high water needs should be closely monitored. The long-term forecast, at the time of writing, calls for a return to warm temperatures and increasing humidity.
Pests/Problems: Wood-rotting fungal pathogens continue to appear, such as Ganoderma sessile on various hardwoods, the northern tooth fungus (Climacodon) on sugar maple and chicken of the wood (Laetiporus) on oaks. Maple anthracnose continues to be a problem on a variety of maples but growth and development has slowed. Birch anthracnose is also abundant, thinning out canopies even in full sun settings. Shaded crabapples on the UMass campus have upwards of 50-75% leaf loss right now due to apple scab. Boxwood blight has made a resurgence with the prolific rainfall in July. Symptoms of the disease include black-colored leaf spots and stem lesions, shedding of foliage from the canopy and rapid death of infected branches. The shedding of foliage is a key symptom to note, as Volutella blight will result in brown leaves that are held in the canopy. Lacebug infestations are becoming visible on both evergreen and deciduous azaleas. When flecking symptoms are present, scout the underside of the foliage to confirm presence of the adult lacebugs. Many late season foliar diseases of deciduous hardwoods, such as Tubakia leaf blotch of oak and Septoria leaf spot of birch may start to appear. While many of these pathogens invade the foliage in the spring, symptom development is delayed for weeks to months. As the majority of new growth is complete for the year, these diseases have a minor impact on overall health. Oriental, Japanese and Asiatic garden beetles are fading as females retreat to the soil to lay eggs. While not the ideal time for transplanting, with proper watering through the end of the growing season it can be successful at this time.
Berkshire Region (Great Barrington)
General Conditions: It has been a wet and wild ride in the Berkshires since June 29 when heavy rains and high winds began more than a month-long stretch of almost daily rain and several damaging wind events. Rainfall for July set all-time records for several towns. Rainfall at official National Weather Service sites were as follows: North Adams with 12.17 inches, Richmond with 12.47 inches, and Pittsfield with 10.75, though the weather recording instruments at the Pittsfield Airport (location of the Weather Service data collection site) were knocked out for 2 days by a lightning strike. It is estimated that 3 more inches of rain fell in Pittsfield during those 3 days, bringing Pittsfield’s total to 13.75 inches. Rainfall gathered in my rain gauge in West Stockbridge amounted to 14.84 inches. A private resident in Lee reported 16 inches in his rain gauge. Needless to say, though rainfall amounts varied amid the hills and valleys of the county, July was extremely wet with only a few days free of precipitation. It was also a cool month most of the time with several early morning thermometer readings dipping into the mid to upper 40s. A drier and somewhat warmer trend seems to be taking hold this week. There were also several high wind events, including several microbursts in the Lenox area on July 27. Estimated winds of 80- to 90 miles per hour raced along narrow strips 50-100 yards wide for a half mile or so in several locations near the downtown area and at the Pleasant Valley Wildlife Sanctuary. Dozens, if not hundreds of trees, were taken down by the hurricane force winds. The multiple downpours during the month also caused much flooding, road and bridge destruction, and considerable erosion. It was a month for the record books. Soils, for the most part, remain saturated or nearly so.
Pests/Problems: Besides the obvious loss of trees and the damage to many of those which are still standing, there are many other visible effects of the persistent wet weather. Foliar diseases are particularly prominent. Diseases such as apple scab have practically denuded ornamental crabapple trees. Powdery mildew is one of the dominant foliar diseases of herbaceous perennials, especially Monarda species and cultivars, and some woody species such as lilac. Also visible is the yellowing of foliage on many woody and herbaceous plants due to nitrogen deficiencies which in turn have been caused by excessive leaching or due to root damage. Another symptom of root damage is the wilting of foliage on many plants in areas that were flooded for days. Soil erosion on sloped sites also exposed roots of some plants. Pest wise, Japanese and Asiatic garden beetles populations have declined. However, it was observed that some trees and shrubs which had been denuded by Lymantria dispar earlier this year and have recently put out another set of leaves, suffered much damage to that new foliage by these beetles. With the frequency of puddling due to frequent rain, the mosquito population has become a major nuisance.
Regional Scouting Credits
- CAPE COD REGION - Russell Norton, Horticulture and Agriculture Educator with Cape Cod Cooperative Extension, reporting from Barnstable.
- SOUTHEAST REGION - Brian McMahon, Arborist, reporting from the Dighton area.
- NORTH SHORE REGION - Geoffrey Njue, Green Industry Specialist, UMass Extension, reporting from the Long Hill Reservation, Beverly.
- EAST REGION - Kit Ganshaw & Sue Pfeiffer, Horticulturists reporting from the Boston area.
- METRO WEST REGION – Julie Coop, Forester, Massachusetts Department of Conservation & Recreation, reporting from Acton.
- CENTRAL REGION - Mark Richardson, Director of Horticulture reporting from Tower Hill Botanic Garden, Boylston.
- PIONEER VALLEY REGION - Nick Brazee, Plant Pathologist, UMass Extension Plant Diagnostic Lab, reporting from Amherst.
- BERKSHIRE REGION - Ron Kujawski, Horticultural Consultant, reporting from Great Barrington.
Woody Ornamentals
Diseases
Recent pests and pathogens of interest seen in the UMass Extension Plant Diagnostic Lab https://ag.umass.edu/services/plant-diagnostics-laboratory:
- Mimosa wilt caused by Fusarium oxysporum f.sp. perniciosum on mimosa/silk tree (Albizia julibrissin). The tree is 2” in diameter and has been present at the site for two years. It was planted in a sandy-loam soil with full sun and no supplemental water. Over the past month, foliage has been shedding from declining branches and epicormic sprouts are forming on the main stem. A few branches remain healthy. Mimosa wilt is a serious disease of Albizia and infections often lead to serious dieback and death of the tree. Early to mid-summer, depending on local conditions, leaves begin to wilt, become yellow/brown and are shed from the canopy. The symptoms most often progress branch by branch over the course of several weeks, but in some cases, larger portions of the canopy become symptomatic and die together. Water sprouts may develop on the lower trunk and sap/gum may ooze from main branches and the trunk as the tree dies. A white, frothy exudate can weep from cracks on the trunk and this is sometimes known as alcoholic flux. Brown vascular streaks may also be present in stems and branches during the early stages of symptom development. The pathogen overwinters in the soil and invades through fine roots in the spring (similar to Verticillium wilt). Once present in the vascular tissue, it is transported with the water stream throughout the canopy of the tree. Large masses of spores are produced on infected shoots and leaves and these are washed down to the soil where the pathogen produces a hardy, overwintering structure (chlamydospore). The pathogen is specific to Albizia and does not present a risk to other nearby trees of different genera.
- Bacterial leaf spot of ginkgo (Ginkgo biloba) caused by Pseudomonas syringae. The tree is approximately 100-years-old and resides in full sun with compacted soils. The tree has suffered from previous unspecified construction-related damage. In July, anthracnose-like symptoms developed in the canopy. On ginkgo, Pseudomonas is capable of causing both leaf spots and stem cankers. The leaf spots are small, rectangular in shape and appear orange-brown with yellow halos. Stem cankering was not present on the submitted sample. Abundant precipitation in July likely enabled disease development and spread throughout the canopy. Overall, the disease is not considered a serious threat to long-term health.

 Dogwood powdery mildew caused by Erysiphe pulchra on flowering dogwood (Cornus florida). The tree is >20-years-old and has been present on the property for nearly as long. The tree resides in a small, residential yard adjacent to a sidewalk with full sun and no supplemental irrigation. As a mature tree, the canopy is fairly dense. This summer, curling and cupping of the foliage at the shoot tips was observed. While dogwoods have naturally curled and drooping leaves, this pattern of distortion was notable and primarily occurred on the newest growth (as seen in the photo below). Dogwoods exhibit indeterminate growth, which means that new shoots and leaves develop throughout the course of the growing season should conditions allow. The abundant rainfall in July allowed many trees with indeterminate growth (others include crabapple, hemlock, black birch) to flush new growth. However, this immature foliage was highly susceptible to powdery mildew infection. Normally, leaves fully mature before PM fungi are actively causing disease, which is why the disease is more serious this season on many landscape dogwoods. While difficult to see in the field, microscopic evaluation revealed a fine, web-like layer of fungal mycelia across the surface of the leaves, in addition to small, black-colored fruiting bodies. The lab has seen an increase in dogwood PM samples over the past two years, with surprising levels of damage in some cases.
Dogwood powdery mildew caused by Erysiphe pulchra on flowering dogwood (Cornus florida). The tree is >20-years-old and has been present on the property for nearly as long. The tree resides in a small, residential yard adjacent to a sidewalk with full sun and no supplemental irrigation. As a mature tree, the canopy is fairly dense. This summer, curling and cupping of the foliage at the shoot tips was observed. While dogwoods have naturally curled and drooping leaves, this pattern of distortion was notable and primarily occurred on the newest growth (as seen in the photo below). Dogwoods exhibit indeterminate growth, which means that new shoots and leaves develop throughout the course of the growing season should conditions allow. The abundant rainfall in July allowed many trees with indeterminate growth (others include crabapple, hemlock, black birch) to flush new growth. However, this immature foliage was highly susceptible to powdery mildew infection. Normally, leaves fully mature before PM fungi are actively causing disease, which is why the disease is more serious this season on many landscape dogwoods. While difficult to see in the field, microscopic evaluation revealed a fine, web-like layer of fungal mycelia across the surface of the leaves, in addition to small, black-colored fruiting bodies. The lab has seen an increase in dogwood PM samples over the past two years, with surprising levels of damage in some cases.- Fruit rot of apple (Malus domestica) caused by an infestation of the apple maggot fly (AM; Rhagoletis pomonella) and secondary invasion by Colletotrichum. The tree is young, approximately 10- to 15-years-old and has been present at the site for more than six years. It resides on the northern slope of an irrigated lawn and receives regular pruning and sanitation. This June, and in previous years, the developing fruit appeared blackened and shriveled or had portions that were browning and rotted. The foliage also had brown spots and blotches with necrosis of the newest growth. The AM is a native fruit fly that became a major pest in the northeast and eastern Canada as commercial apple production developed. In addition to apple, the AM can also infest cherry, plum, peach and pears. However, apple is the primary host for this pest. The tunneling and feeding by the larvae within the fruit destroys them for sale and consumption. Colletotrichum infectionscan result in a fruit rot, foliar blight and stem cankers on a variety of trees and shrubs. It likely colonized wounds made by the AM, where it caused a brown-colored rot of the fruit tissue.

 Fusarium wilt of fragrant sumac (Rhus aromatica ‘Gro-Low’) caused by Fusarium oxysporum. Small, mass planting in a roadside bed on the UMass campus in full sun with no supplemental irrigation. In 2020, symptoms of the disease were observed on plants in the same bed and the pathogen was identified by DNA sequence analysis after isolating F. oxysporum in pure culture. Symptoms of the disease reappeared this summer and included browning foliage, branch dieback and blackening of the vascular tissue. Once Fusarium wilt is present in a bed, fragrant sumac should not be replanted. Drought and heat stress may be predisposing factors.
Fusarium wilt of fragrant sumac (Rhus aromatica ‘Gro-Low’) caused by Fusarium oxysporum. Small, mass planting in a roadside bed on the UMass campus in full sun with no supplemental irrigation. In 2020, symptoms of the disease were observed on plants in the same bed and the pathogen was identified by DNA sequence analysis after isolating F. oxysporum in pure culture. Symptoms of the disease reappeared this summer and included browning foliage, branch dieback and blackening of the vascular tissue. Once Fusarium wilt is present in a bed, fragrant sumac should not be replanted. Drought and heat stress may be predisposing factors.- Dutch elm disease, caused by Ophiostoma novo-ulmi, on American elm (Ulmus americana). The tree is approximately 40-years-old and experiences half sun in sandy soils within a residential backyard. The entire canopy appeared thin in early July, with leaf wilting and shedding. The submitted stems had vascular tissue that was completely brown and after a short incubation, O. novo-ulmi appeared from this diseased tissue. This pattern of symptoms, specifically the entire crown wilting, thinning and shedding foliage, has been frequently observed in 2021. This is in contrast to more typical DED symptoms, which usually manifests as single branch flagging. It's possible that trees were resisting infections and were overtaken by the drought and heat of 2020. This may have allowed the pathogen to become systemic in the tree, moving in the vascular stream to the roots late in the season. A similar pattern of symptoms occurred in 2017, the year following the 2016 drought, when many very large and old “survivor” elms succumbed to DED in the region, including the state champion in Old Deerfield.
 Severe infestation of the euonymus scale (Unaspis euonymi) on evergreen Euonymus (likely E. japonicus). The plant is approximately 20-years-old and resides in a foundation bed with partial sun and supplemental irrigation from lawn sprinklers. The homeowner discovered the infestation in 2020 and tried several sprays (of unknown material) to no avail. Like many armored scale insect pests in the landscape, the euonymus scale is non-native to North America. When populations are high, leaf yellowing, premature leaf shedding and canopy dieback can develop. Female scales are brown in color, oyster shell-shaped and easily camouflaged on the bark while the males are elongated and white.
Severe infestation of the euonymus scale (Unaspis euonymi) on evergreen Euonymus (likely E. japonicus). The plant is approximately 20-years-old and resides in a foundation bed with partial sun and supplemental irrigation from lawn sprinklers. The homeowner discovered the infestation in 2020 and tried several sprays (of unknown material) to no avail. Like many armored scale insect pests in the landscape, the euonymus scale is non-native to North America. When populations are high, leaf yellowing, premature leaf shedding and canopy dieback can develop. Female scales are brown in color, oyster shell-shaped and easily camouflaged on the bark while the males are elongated and white.- Premature needle shedding and stem dieback of weeping white spruce (Picea glauca ‘Pendula’) caused by the spruce spider mite (Oligonychus ununguis), Rhizosphaera needle cast and Phomopsis stem cankering. The tree is approximately five to eight-years-old and has been present at the site for only ten months. It was planted close to a residential home with afternoon sun and no supplemental irrigation. For landscape spruce, this trifecta of mite infestation and diseases can be exceptionally destructive. Management of spruce with similar problems should first focus on eradicating the spider mites, since targeted miticide applications are highly effective. Next, if drought stress is an issue this must be addressed since needle blight pathogens like Rhizosphaera thrive on drought-stressed trees. Pruning and removal of diseased stems is necessary to reduce inoculum. For smaller trees, regular fungicide application can control both Rhizosphaera and Phomopsis, if inoculum is reduced at the site. Weeping white spruce is a phenomenal tree that is underused in the landscape. This vigorous cultivar maintains a tight, weeping canopy along a central leader that requires little maintenance once established.
 Infestation of the black tupelo bladder gall mite (Eriophyes nyssae) on black tupelo (Nyssa sylvatica). The tree is young, less than 10-years-old and was planted in a shaded, south-facing setting with lawn sprinklers providing some supplemental water. This year, the leaf margins appeared curled, swollen and distorted. Eriophyid mites create a range of interesting and bizarre leaf galls on deciduous trees and shrubs. While they cause almost no harm to woody plants, their actions are highly conspicuous and proper identification is useful in that regard. The galls produced by the black tupelo bladder gall mite can create ragged, wavy leaf margins, as shown here. It can also create rounded, green-colored galls on interior portions of the leaf but none were encountered on this sample.
Infestation of the black tupelo bladder gall mite (Eriophyes nyssae) on black tupelo (Nyssa sylvatica). The tree is young, less than 10-years-old and was planted in a shaded, south-facing setting with lawn sprinklers providing some supplemental water. This year, the leaf margins appeared curled, swollen and distorted. Eriophyid mites create a range of interesting and bizarre leaf galls on deciduous trees and shrubs. While they cause almost no harm to woody plants, their actions are highly conspicuous and proper identification is useful in that regard. The galls produced by the black tupelo bladder gall mite can create ragged, wavy leaf margins, as shown here. It can also create rounded, green-colored galls on interior portions of the leaf but none were encountered on this sample.- Branch dieback of thornless honey-locust (Gleditsia triacanthos f. inermis) caused by the fungal pathogens Cytospora and Colletotrichum. The tree is approximately 60-years-old and resides in a full sun, lawn setting with no supplemental irrigation. The managing arborist has no history with the tree and is trying to establish an IPM program for the future. The canopy appears thin and there are numerous dead branches, comprising roughly 25% of the canopy. Colletotrichum is a common anthracnose pathogen of deciduous hardwoods in the region andwas present on the foliage and petioles. For trees with compound leaves like honey-locust, anthracnose infections of the petiole can result in rapid leaf shedding. Meanwhile, Cytospora was present on submitted stems and small branches. Cytospora often functions as an opportunist on stressed and weakened trees.
Report by Nick Brazee, Plant Pathologist, UMass Extension Plant Diagnostic Lab, UMass Amherst.
Insects
Insects and Other Arthropods of Medical Importance:


 Browntail Moth: (Euproctis chrysorrhoea) is an insect that was accidentally introduced to Massachusetts from Europe in 1897. By the early 1900s, it spread into all of New England and parts of Canada. The caterpillars of this species feed on oak, shadbush, cherry, beach plum, apple, rugosa rose, and other trees and shrubs. While the feeding damage from the caterpillars on landscape specimens may be problematic, the primary cause for concern with regard to browntail moth is medical: the caterpillars of this species possess poisonous hairs that cause a rash similar to poison ivy, and, in some sensitive individuals, may cause trouble breathing or sometimes a more severe allergic reaction.
Browntail Moth: (Euproctis chrysorrhoea) is an insect that was accidentally introduced to Massachusetts from Europe in 1897. By the early 1900s, it spread into all of New England and parts of Canada. The caterpillars of this species feed on oak, shadbush, cherry, beach plum, apple, rugosa rose, and other trees and shrubs. While the feeding damage from the caterpillars on landscape specimens may be problematic, the primary cause for concern with regard to browntail moth is medical: the caterpillars of this species possess poisonous hairs that cause a rash similar to poison ivy, and, in some sensitive individuals, may cause trouble breathing or sometimes a more severe allergic reaction.
UMass Extension was recently made aware of reports of adult browntail moths coming to lights in Essex and Plymouth counties, as well as the Boston area (Chestnut Hill). While the presence of adult browntail moth in locations outside of Cape Cod represents a change in the recent historical distribution of this insect in Massachusetts, we do not believe browntail moth populations are elevated or widespread in MA at this time.
However, due to the medical concerns associated with this insect, we are recommending that green industry professionals working in Massachusetts, particularly in coastal areas of Barnstable, Essex, Norfolk, Plymouth, and Suffolk Counties, should review the information provided here and be able to accurately identify this insect in all life stages. Key in this information is the note that they should avoid touching the caterpillars, pupae/cocoons, or the nests/webbing produced by this pest without protective eyewear, gloves, long sleeves, pants, and other protective clothing. (The adult moths themselves do not seem to produce highly irritating hairs, yet the hairs from the caterpillar and pupal stages may remain on the adults after they emerge.)
For more information, visit: https://ag.umass.edu/landscape/news/time-to-reacquaint-yourself-with-browntail-moth
To report suspected browntail moth adults (at present, it is the adult stage of this insect that is active at this time of year) in Massachusetts, visit: https://massnrc.org/pests/pestreports.htm
- Mosquitoes: The Massachusetts Department of Public Health announced on July 1, 2021 that West Nile virus (WNV) was detected in mosquitoes in Massachusetts for the first time this year in a sample collected on June 29 in the town of Medford, MA (Middlesex County). To date, no human or animal cases of WNV or Eastern Equine Encephalitis (EEE) have been detected so far in 2021. For more information, visit: https://www.mass.gov/news/state-public-health-officials-confirm-seasons-first-west-nile-virus-positive-mosquito-sample AND https://www.mass.gov/info-details/massachusetts-arbovirus-update .
According to the Massachusetts Bureau of Infectious Disease and Laboratory Science and the Department of Public Health, there are at least 51 different species of mosquito found in Massachusetts. Mosquitoes belong to the Order Diptera (true flies) and the Family Culicidae (mosquitoes). As such, they undergo complete metamorphosis, and possess four major life stages: egg, larva, pupa, and adult. Adult mosquitoes are the only stage that flies and many female mosquitoes only live for 2 weeks (although the life cycle and timing will depend upon the species). Only female mosquitoes bite to take a blood meal, and this is so they can make eggs. Mosquitoes need water to lay their eggs in, so they are often found in wet or damp locations and around plants. Different species prefer different habitats. It is possible to be bitten by a mosquito at any time of the day, and again timing depends upon the species. Many are particularly active from just before dusk, through the night, and until dawn. Mosquito bites are not only itchy and annoying, but they can be associated with greater health risks. Certain mosquitoes vector pathogens that cause diseases such as West Nile virus (WNV) and eastern equine encephalitis (EEE).
For more information about mosquitoes in Massachusetts, visit: https://www.mass.gov/service-details/mosquitoes-in-massachusetts
There are ways to protect yourself against mosquitoes, including wearing long-sleeved shirts and long pants, keeping mosquitoes outside by using tight-fitting window and door screens, and using insect repellents as directed. Products containing the active ingredients DEET, permethrin, IR3535, picaridin, and oil of lemon eucalyptus provide protection against mosquitoes.
For more information about mosquito repellents, visit: https://www.mass.gov/service-details/mosquito-repellents and https://www.cdc.gov/mosquitoes/mosquito-bites/prevent-mosquito-bites.html .

 American Dog Tick: Anecdotally, Dermacentor variabilis has been prevalent in certain locations in Massachusetts in 2021. The images shown here are adult stage dog ticks removed from a dog following a roadside walk in Hampshire County on both 5/6/21 (4 ticks removed) and 5/7/21 (3 ticks removed). Looking for more updates? Check out UMass Extension’s Tick Check with Blake Dinius, Plymouth County Extension Service and Larry Dapsis, Cape Cod Cooperative Extension: https://www.youtube.com/watch?v=gryCv8qB1qw .
American Dog Tick: Anecdotally, Dermacentor variabilis has been prevalent in certain locations in Massachusetts in 2021. The images shown here are adult stage dog ticks removed from a dog following a roadside walk in Hampshire County on both 5/6/21 (4 ticks removed) and 5/7/21 (3 ticks removed). Looking for more updates? Check out UMass Extension’s Tick Check with Blake Dinius, Plymouth County Extension Service and Larry Dapsis, Cape Cod Cooperative Extension: https://www.youtube.com/watch?v=gryCv8qB1qw .
The American dog tick is found throughout most of North America. It may be encountered in forest edges, fields, along walkways and roadways, sidewalks, and trails. Adult stage ticks may be found on raccoons, skunks, cats, dogs, and other medium-sized hosts. Larvae and nymphs can be found on mice, voles, rats, and chipmunks. Adult males and females are active between April and early-August. Both adult males and females will feed, including on people. Nymphs and larvae of this species rarely attach to people or their pets. This species of tick can transmit lesser-known diseases such as Rocky Mountain Spotted Fever (not frequently infecting humans, according to CDC reports) and Tularemia (rarely infecting humans, according to CDC reports). For more information about the American dog tick, visit: https://web.uri.edu/tickencounter/species/dog-tick/ .
- Deer Tick/Blacklegged Tick: Check out the archived TickTalk with TickReport webinars available here: https://ag.umass.edu/landscape/education-events/webinars .
*Ixodes scapularis - We are now in the time of year when deer tick larvae and nymphs are frequently encountered. Larvae may be encountered in April, but in some locations may peak in their activity in August, while still being encountered through November. Nymphs are encountered from April through July, peaking in June. Nymphs are again present in October and November. For images of all deer tick life stages, along with an outline of the diseases they carry, and their timing of activity, visit: http://www.tickencounter.org/tick_identification/deer_tick .
Anyone working in the yard and garden should be aware that there is the potential to encounter deer ticks. The deer tick or blacklegged tick can transmit Lyme disease, human babesiosis, human anaplasmosis, and other diseases. Preventative activities, such as daily tick checks, wearing appropriate clothing, and permethrin treatments for clothing (according to label instructions) can aid in reducing the risk that a tick will become attached to your body. If a tick cannot attach and feed, it will not transmit disease. For more information about personal protective measures, visit: http://www.tickencounter.org/prevention/protect_yourself .
The Center for Agriculture, Food, and the Environment provides a list of potential tick identification and testing resources here: https://ag.umass.edu/resources/tick-testing-resources
*Note that deer ticks (Ixodes scapularis) are not the only disease-causing tick species found in Massachusetts. The American dog tick (Dermacentor variabilis) and the lone star tick (Amblyomma americanum) are also found throughout MA. Each can carry their own complement of diseases, including others not mentioned above. Anyone working or playing in tick habitats (wood-line areas, forested areas, and landscaped areas with ground cover) should check themselves regularly for ticks while practicing preventative measures.

 Wasps/Hornets: Many wasps are predators of other arthropods, including pest insects such as certain caterpillars that feed on trees and shrubs. Adult wasps hunt prey and bring it back to their nest where young are being reared, as food for the immature wasps. A common such example are the paper wasps (Polistes spp.) who rear their young on chewed up insects. They may be seen searching plants for caterpillars and other soft-bodied larvae to feed their young. Paper wasps can sting, and will defend their nests, which are open-celled paper nests that are not covered with a papery “envelope”. These open-celled nests may be seen hanging from eaves or other outdoor building structures. Aerial yellow jackets and hornets create large aerial nests that are covered with a papery shell or “envelope”. Common yellow jacket species include those in the genus Vespula. Dolichovespula maculata is commonly known as the baldfaced hornet, although it is not a true hornet. The European hornet (Vespa crabro) is three times the size of a yellow jacket and may be confused for the Asian giant hornet (Vespa mandarinia). The European hornet is known to Massachusetts, but the Asian giant hornet is not. If you are concerned that you have found or photographed an Asian giant hornet, please report it here: https://massnrc.org/pests/report.aspx . Paper wasps and aerial yellowjackets overwinter as fertilized females (queens) and a single female produces a new nest annually in the late spring. Nests are abandoned at the end of the season. Queens start new nests, lay eggs, and rear new wasps to assist in colony/nest development.Some people are allergic to stinging insects, so care should be taken around wasp/hornet nests. Unlike the European honeybee (Apis mellifera), wasps and hornets do not have barbed stingers, and therefore can sting repeatedly when defending their nests. It is best to avoid their nests, and if that cannot be done and assistance is needed to remove them, consult a professional.
Wasps/Hornets: Many wasps are predators of other arthropods, including pest insects such as certain caterpillars that feed on trees and shrubs. Adult wasps hunt prey and bring it back to their nest where young are being reared, as food for the immature wasps. A common such example are the paper wasps (Polistes spp.) who rear their young on chewed up insects. They may be seen searching plants for caterpillars and other soft-bodied larvae to feed their young. Paper wasps can sting, and will defend their nests, which are open-celled paper nests that are not covered with a papery “envelope”. These open-celled nests may be seen hanging from eaves or other outdoor building structures. Aerial yellow jackets and hornets create large aerial nests that are covered with a papery shell or “envelope”. Common yellow jacket species include those in the genus Vespula. Dolichovespula maculata is commonly known as the baldfaced hornet, although it is not a true hornet. The European hornet (Vespa crabro) is three times the size of a yellow jacket and may be confused for the Asian giant hornet (Vespa mandarinia). The European hornet is known to Massachusetts, but the Asian giant hornet is not. If you are concerned that you have found or photographed an Asian giant hornet, please report it here: https://massnrc.org/pests/report.aspx . Paper wasps and aerial yellowjackets overwinter as fertilized females (queens) and a single female produces a new nest annually in the late spring. Nests are abandoned at the end of the season. Queens start new nests, lay eggs, and rear new wasps to assist in colony/nest development.Some people are allergic to stinging insects, so care should be taken around wasp/hornet nests. Unlike the European honeybee (Apis mellifera), wasps and hornets do not have barbed stingers, and therefore can sting repeatedly when defending their nests. It is best to avoid their nests, and if that cannot be done and assistance is needed to remove them, consult a professional.
Woody ornamental insect and non-insect arthropod pests to consider, a selected few:
Invasive Insects & Other Organisms Update:
![Box tree moth adult. (Matteo Maspero and Andrea Tantardini, Centro MiRT Fondazione Minoprio [IT]) Box tree moth adult. (Matteo Maspero and Andrea Tantardini, Centro MiRT Fondazione Minoprio [IT])](/sites/ag.umass.edu/files/styles/150x150/public/pest-alerts/images/content/box_tree_moth_courtesy_of_matteo_maspero_and_andrea_tantardini_centro_mirt_fondazione_minoprio_it.jpg?itok=pIFkiiQi)
![Box tree moth caterpillars. (Matteo Maspero and Andrea Tantardini, Centro MiRT Fondazione Minoprio [IT]) Box tree moth caterpillars. (Matteo Maspero and Andrea Tantardini, Centro MiRT Fondazione Minoprio [IT])](/sites/ag.umass.edu/files/styles/150x150/public/pest-alerts/images/content/box_tree_moth_caterpillar_courtesy_of_matteo_maspero_and_andrea_tantardini_centro_mirt_fondazione_minoprio_it.jpg?itok=BDTKHKwy) Box Tree Moth: Cydalima perspectalis is native to East Asia. It has become a serious invasive pest in Europe, where it continues to spread. The caterpillars feed mostly on boxwood, and heavy infestations can defoliate host plants. Once the leaves are gone, larvae consume the bark, leading to girdling and plant death. The box tree moth is not currently known to be established in Massachusetts, however the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) is urging professionals and citizens to report any suspicious insects.
Box Tree Moth: Cydalima perspectalis is native to East Asia. It has become a serious invasive pest in Europe, where it continues to spread. The caterpillars feed mostly on boxwood, and heavy infestations can defoliate host plants. Once the leaves are gone, larvae consume the bark, leading to girdling and plant death. The box tree moth is not currently known to be established in Massachusetts, however the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) is urging professionals and citizens to report any suspicious insects.
To report suspected box tree moth life stages or damage to boxwood in Massachusetts, please visit: https://massnrc.org/pests/pestreports.htm .
Females lay eggs singly or in clusters of 5 to more than 20 eggs in a gelatinous mass on the underside of boxwood leaves. Most females deposit more than 42 egg masses in their lifetime. They typically hatch within 4 to 6 days. Pupae typically first appear in April or May and are present continuously through the summer and into the fall, depending on the local climate and timing of generations. Adults first emerge from the overwintering generation between April and July, depending on climate and temperature. Subsequent generations are active between June and October. Adults typically live for two weeks after emergence. The exact timing of the life cycle of this insect in Massachusetts is not currently known.
The Massachusetts Department of Agricultural Resources (MDAR) recently reported that the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) has confirmed the presence of box tree moth in the continental United States and is taking action alongside state partners and industry to contain and eradicate the invasive pest that was imported on nursery plants shipped from Ontario, Canada. For a recent press release regarding this insect from the MA Department of Agricultural Resources, visit: https://www.mass.gov/news/state-agricultural-officials-urge-public-to-inspect-boxwood-shrubs-for-box-tree-moths .
Between August 2020 and April 2021, a nursery in St. Catharines, Ontario shipped boxwood (Buxus species) that may have been infested with box tree moth to locations in six states—25 retail facilities in Connecticut, Massachusetts, Michigan, New York, Ohio, and South Carolina—and a distribution center in Tennessee. At this time, the pest has been identified in three facilities in Michigan, one in Connecticut, and one in South Carolina, and APHIS is working with state plant regulatory officials to determine whether other facilities may be impacted. For more information, visit: https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-05/box-tree-moth .
 Hemlock Woolly Adelgid: Adelges tsugae is an invasive pest found on eastern and Carolina hemlock. Hemlock woolly adelgid populations were reportedly very high in certain locations across Massachusetts and much of New England this year. UMass Extension has fielded many reports of infested trees and questions about HWA management. A fascinating story about hemlock woolly adelgid has developed and was recently shared with UMass Extension by our friends at the Maine Forest Service:
Hemlock Woolly Adelgid: Adelges tsugae is an invasive pest found on eastern and Carolina hemlock. Hemlock woolly adelgid populations were reportedly very high in certain locations across Massachusetts and much of New England this year. UMass Extension has fielded many reports of infested trees and questions about HWA management. A fascinating story about hemlock woolly adelgid has developed and was recently shared with UMass Extension by our friends at the Maine Forest Service:
In June, 2021, news articles began appearing regarding a strange phenomenon of beachgoers in southern Maine experiencing their bare feet being stained black as they walked along the beach. (See photos in the article: “What’s staining the feet of southern Maine beachgoers? DEP officials investigating.” by Steven Porter and Camille Fine, Portsmouth Herald on June 9, 2021.) Well, the Maine Forest Service released in their July 22, 2021 Conditions Report under “Insects” the following statement:
“Remember that bizarre story about beach-goers in Southern Maine going home with stained and blackened feet? Well, there’s a new plot twist – it seems that the billions of tiny insects washing up on our beaches were none other than hemlock woolly adelgid (HWA) adults.
When we first received samples of the insects from Wells Beach, we weren’t quite sure what they were. Once we saw them, our original sight-unseen hypothesis that they might be a species of kelp fly went right out the window. These tiny insects were clearly true bugs and had two pairs of wings, immediately disqualifying any sort of fly. Karen Coluzzi, in the Cooperative Agricultural Pest Survey program, initially suggested Adelgidae, which was confirmed by USFS. We sent a sample to Nathan Havill for DNA-barcoding and after several inconclusive results from the deteriorating sample, a clear read came back with a clear answer – these weren’t any of our other common native adelgids, but the invasive HWA.
Many adelgids have complex life cycles with multiple forms, including both winged (alate) and non-winged forms. In its native range, HWA has multiple host trees, and the winged adults are those that leave hemlock trees to go in search of certain spruce species, which serves as their primary host species. In North America these particular spruce species are absent, so the good news is, this life stage is a dead end.”
The entire Conditions Report from the Maine Forest Service is available here: https://content.govdelivery.com/accounts/MEDACF/bulletins/2e96444 Thank you Maine Forest Service for sharing such an interesting story!
Additionally, Dr. David Orwig (Forest Ecologist, Harvard Forest) shared that he experienced sexuparae (winged HWA) flights on June 11, 2021 in Petersham, MA where HWA populations were also very high this year. He noted that the winged individuals quickly coated the front of a vehicle. (Personal Communication.) Some news reports also indicate that this phenomenon might have been experienced at beaches in coastal parts of Massachusetts, including Gloucester and Beverly, MA.
HWA Biology/Life Cycle Reminders: The overwintering hemlock woolly adelgid generation (sistens) is present through mid-spring and produces the spring generation (progrediens) which is present from early spring through mid-summer. HWA, unlike many other insects, does most of its feeding over the winter. Eggs may be found in woolly masses at the base of hemlock needles beginning in mid-March. Each woolly mass is created by a female who may then lay 50-300 eggs. Eggs hatch and crawlers may be found from mid-March through mid-July. Infested trees may be treated with foliar sprays in late April to early May, using Japanese quince as a phenological indicator. Systemic applications may be made in the spring and fall, or when soil conditions are favorable for translocation to foliage. Nitrogen fertilizer applications may make hemlock woolly adelgid infestations worse.
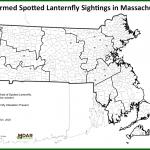 Spotted Lanternfly: (Lycorma delicatula, SLF) is not known to be established in Massachusetts landscapes at this time. However, due to the great ability of this insect to hitchhike using human-aided movement, it is important that we remain vigilant in Massachusetts and report any suspicious findings. Spotted lanternfly reports can be sent here: https://massnrc.org/pests/slfreport.aspx .
Spotted Lanternfly: (Lycorma delicatula, SLF) is not known to be established in Massachusetts landscapes at this time. However, due to the great ability of this insect to hitchhike using human-aided movement, it is important that we remain vigilant in Massachusetts and report any suspicious findings. Spotted lanternfly reports can be sent here: https://massnrc.org/pests/slfreport.aspx .
The Massachusetts Department of Agricultural Resources has recently released spotted lanternfly Best Management Practices for Nurseries and Landscapers: https://massnrc.org/pests/linkeddocuments/MANurseryBMPs.pdf
And Best Management Practices for Moving Companies and the Moving Industry: https://massnrc.org/pests/linkeddocuments/SLFChecklistMovingIndustryMA.pdf
Now is a great time to provide copies of these BMP’s to employees, customers, family, and friends! The more eyes we have out there looking for spotted lanternfly, the better. Use the above BMP’s as a guide to help you inspect certain items coming from CT, DE, MD, NC, NJ, NY, OH, PA, WV, and VA.
UMass Extension is teaming up with UMass Amherst’s Department of Environmental Conservation, the USDA APHIS, and the Massachusetts Department of Agricultural Resources to monitor for the spotted lanternfly in Massachusetts. A team including members of UMass Extension’s Landscape, Nursery, and Urban Forestry Program, Extension’s Fruit Program, Stockbridge School of Agriculture, and the Department of Environmental Conservation at UMass, Amherst are undertaking a nine-month integrated research and extension project to develop effective tools to detect the spotted lanternfly.
The researchers associated with this project (Dr. Joseph Elkinton, Dr. Jeremy Andersen and Dr. Jaime Pinero) will be working with Dr. Miriam Cooperband of the USDA APHIS lab on Cape Cod to identify and evaluate airborne attractants that can improve the ability to detect SLF in traps. Dr. Cooperband has identified several attractant lures released from host plants of SLF. She is currently working on pheromones produced by SLF that may be much more attractive. The UMass team will help her conduct field tests of these new lures, while also assisting the Massachusetts Department of Agricultural Resources (MDAR) in monitoring for SLF in Massachusetts. UMass Extension Entomologist, Tawny Simisky, will periodically report on progress made during the course of this project. For more information, please visit: https://ag.umass.edu/cafe/news/looking-for-spotted-lanternfly-recent-invasive-arrival .
This insect is a member of the Order Hemiptera (true bugs, cicadas, hoppers, aphids, and others) and the Family Fulgoridae, also known as planthoppers. The spotted lanternfly is a non-native species first detected in the United States in Berks County, Pennsylvania and confirmed on September 22, 2014.
For a map of known, established populations of SLF as well as detections outside of these areas where individual finds of spotted lanternfly have occurred (but no infestations are present), visit: https://nysipm.cornell.edu/environment/invasive-species-exotic-pests/spotted-lanternfly/ .
The spotted lanternfly is considered native to China, India, and Vietnam. It has been introduced as a non-native insect to South Korea and Japan, prior to its detection in the United States. In South Korea, it is considered invasive and a pest of grapes and peaches. The spotted lanternfly has been reported feeding on over 103 species of plants, according to new research (Barringer and Ciafré, 2020) and when including not only plants on which the insect feeds, but those that it will lay egg masses on, this number rises to 172. This includes, but is not limited to, the following: tree of heaven (Ailanthus altissima) (preferred host), apple (Malus spp.), plum, cherry, peach, apricot (Prunus spp.), grape (Vitis spp.), pine (Pinus spp.), pignut hickory (Carya glabra), sassafras (Sassafras albidum), serviceberry (Amelanchier spp.), slippery elm (Ulmus rubra), tulip poplar (Liriodendron tulipifera), white ash (Fraxinus americana), willow (Salix spp.), American beech (Fagus grandifolia), American linden (Tilia americana), American sycamore (Platanus occidentalis), big-toothed aspen (Populus grandidentata), black birch (Betula lenta), black cherry (Prunus serotina), black gum (Nyssa sylvatica), black walnut (Juglans nigra), dogwood (Cornus spp.), Japanese snowbell (Styrax japonicus), maple (Acer spp.), oak (Quercus spp.), and paper birch (Betula papyrifera).
The adults and immatures of this species damage host plants by feeding on sap from stems, leaves, and the trunks of trees. In the springtime in Pennsylvania (late April - mid-May) nymphs (immatures) are found on smaller plants and vines and new growth of trees and shrubs. Third and fourth instar nymphs migrate to the tree of heaven and are observed feeding on trunks and branches. Trees may be found with sap weeping from the wounds caused by the insect’s feeding. The sugary secretions (excrement) created by this insect may coat the host plant, later leading to the growth of sooty mold. Insects such as wasps, hornets, bees, and ants may also be attracted to the sugary waste created by the lanternflies, or sap weeping from open wounds in the host plant. Host plants have been described as giving off a fermented odor when this insect is present.
Adults are present by the middle of July in Pennsylvania and begin laying eggs by late September and continue laying eggs through late November and even early December in that state. Adults may be found on the trunks of trees such as the tree of heaven or other host plants growing in close proximity to them. Egg masses of this insect are gray in color and look similar in some ways to gypsy moth egg masses.
Host plants, bricks, stone, lawn furniture, recreational vehicles, and other smooth surfaces can be inspected for egg masses. Egg masses laid on outdoor residential items such as those listed above may pose the greatest threat for spreading this insect via human aided movement.
For more information about the spotted lanternfly, visit this fact sheet: https://ag.umass.edu/landscape/fact-sheets/spotted-lanternfly .
- Emerald Ash Borer: (Agrilus planipennis, EAB) in 2021 alone, the Massachusetts Department of Conservation and Recreation has confirmed at least 28 new community detections of emerald ash borer in Massachusetts. To date, 11 out of the 14 counties in Massachusetts have confirmed emerald ash borer. (The remaining counties where EAB has yet to be detected are Barnstable, Dukes, and Nantucket counties.)A map of these locations and others previously known across the state may be found here: https://ag.umass.edu/fact-sheets/emerald-ash-borer .
This wood-boring beetle readily attacks ash (Fraxinus spp.) including white, green, and black ash and has also been found developing in white fringe tree (Chionanthus virginicus) and has been reported in cultivated olive (Olea europaea). Signs of an EAB infested tree may include D-shaped exit holes in the bark (from adult emergence), “blonding” or lighter coloration of the ash bark from woodpecker feeding (chipping away of the bark as they search for larvae beneath), and serpentine galleries visible through splits in the bark, from larval feeding beneath. It is interesting to note that woodpeckers are capable of eating 30-95% of the emerald ash borer larvae found in a single tree (Murphy et al. 2018). Unfortunately, despite high predation rates, EAB populations continue to grow.
For further information about this insect, please visit: https://ag.umass.edu/fact-sheets/emerald-ash-borer . If you believe you have located EAB-infested ash trees, particularly in an area of Massachusetts not identified on the map provided, please report here: https://massnrc.org/pests/eabreport.htm .


 Lymantria dispar: (Formerly Gypsy Moth; LD) Professionals working in parts of Berkshire County (ex. Alford, Great Barrington, Richmond, Sheffield, South Egremont, and Williamstown) as well as NY and CT report being deluged with questions from property owners looking to manage Lymantria dispar caterpillars in 2022 following expanding populations of this insect in those areas this season. Here is some information to help with those discussions:
Lymantria dispar: (Formerly Gypsy Moth; LD) Professionals working in parts of Berkshire County (ex. Alford, Great Barrington, Richmond, Sheffield, South Egremont, and Williamstown) as well as NY and CT report being deluged with questions from property owners looking to manage Lymantria dispar caterpillars in 2022 following expanding populations of this insect in those areas this season. Here is some information to help with those discussions:
In high populations of Lymantria dispar (formerly gypsy moth), scraping egg masses can be a futile effort. When populations are high, caterpillars can blow in from surrounding forested areas onto your property (next spring) even after an egg mass scraping effort is undertaken. Additionally, on large trees, female moths can lay their egg masses in areas that are not practical/safe to reach. However, if you do try to remove egg masses from high value specimen trees this fall and winter, be sure to scrape them into a can of soapy water. If scraped onto the ground, they may still hatch next spring.
A good plan would be to have an arborist come monitor the property and surrounding area this fall/winter once Lymantria dispar females are completely done laying eggs. (Additional egg mass survey information will also be available for Massachusetts through the Department of Conservation and Recreation once surveys are complete for 2021, here: ( https://www.mass.gov/guides/lymantria-dispar-gypsy-moth-in-massachusetts ). If there are large numbers of egg masses on your property and in the woods nearby, consider applying reduced risk insecticides next spring to protect high-value specimen trees from defoliation next year, especially if they could become hazardous if they were to decline. (Ex. trees near the home, garage, etc.) Applications would be made following 90-100 GDD's, after eggs have hatched, and caterpillars have settled to feed. Egg hatch begins roughly around the first week in May in MA, however this timing may depend upon spring temperatures. Special care should also be taken to protect trees that were defoliated this year, as two consecutive years of defoliation are often very stressful even for mature trees. Young trees/new plantings should be protected as well.
After caterpillars hatch, and once they settle to begin feeding (when they are approximately 3/4 inch in length or less), they are very susceptible to applications of the reduced risk insecticide known as Bacillus thuringiensis Kurstaki. This is a soil dwelling bacterium that is lethal to the caterpillars if they ingest it on the leaves. Another reduced risk option is the active ingredient spinosad (also derived from a soil dwelling bacterium). Spinosad should not be applied to plants in bloom, as it is toxic to pollinators - but that toxicity goes away once the product dries (in about 3 hours). Chlorantraniliprole is another reduced risk active ingredient that can be applied to the leaves of susceptible hosts when caterpillars are young and just beginning to feed.
The efficacy of systemic insecticides for the management of Lymantria dispar is not entirely understood. However, products containing azadirachtin, abamectin, or acephate are labelled for use against this insect. Of those, azadirachtin is a reduced risk insecticide. With regard to acephate, some research suggests that it is more effective at managing caterpillars feeding on smaller diameter trees than on larger trees (Dan Herms, Personal Communication).
LD moth has been in Massachusetts since the 1860's. This invasive insect from Europe often goes unnoticed, thanks to population regulation provided by the entomopathogenic fungus, E. maimaiga, as well as a NPV virus specific to LD moth caterpillars. (And to a lesser extent many other organisms, including other insects, small mammals, and birds who feed on LD moth.) However, if environmental conditions do not favor the life cycle of the fungus, outbreaks of LD moth caterpillars are possible. (Such as most recently from 2015-2018, with a peak in the LD moth population in 2017 in Massachusetts.)
Check out Episode 1 of InsectXaminer to reminisce about the 2015-2018 outbreak of this insect and learn more about the fungus and the virus and how to recognize caterpillars that have been killed by these pathogens: https://ag.umass.edu/landscape/education-events/insectxaminer .
- Asian Longhorned Beetle: (Anoplophora glabripennis, ALB) Look for signs of an ALB infestation which include perfectly round exit holes (about the size of a dime), shallow oval or round scars in the bark where a female has chewed an egg site, or sawdust-like frass (excrement) on the ground nearby host trees or caught in between branches. Be advised that other, native insects may create perfectly round exit holes or sawdust-like frass, which can be confused with signs of ALB activity.
The regulated area for Asian longhorned beetle is 110 miles2 encompassing Worcester, Shrewsbury, Boylston, West Boylston, and parts of Holden and Auburn. If you believe you have seen damage caused by this insect, such as exit holes or egg sites, on susceptible host trees like maple, please call the Asian Longhorned Beetle Eradication Program office in Worcester, MA at 508-852-8090 or toll free at 1-866-702-9938.
To report an Asian longhorned beetle find online or compare it to common insect look-alikes, visit: http://massnrc.org/pests/albreport.aspx or https://www.aphis.usda.gov/pests-diseases/alb/report .
- Jumping Worms: In recent years, public concern about Amynthas spp. earthworms, collectively referred to as “jumping or crazy or snake” worms, has dramatically increased. University researchers and Extension groups in many locations in the US are finding that these species cause not only forest ecosystem disturbances, but may also negatively impact soil structure and reduce plant growth in gardens and managed landscapes. They do this by voraciously devouring the organic layer of the soil while feeding very close to the soil surface, unlike other species of earthworms. In woodland areas, they can quickly eat all of the leaf litter on the forest floor. Jumping worms also leave a distinct grainy soil full of worm castings. The soil becomes granular and may look like dried coffee grounds.
Unfortunately, there are currently no research-based management options available for these earthworms. So prevention is essential – preventing their introduction and spread into new areas is the best defense against them. Adult jumping worms can be 1.5 – 8 inches or more in length. Their clitellum (collar-like ring) is roughly located 1/3 down the length of the worm (from the head) and is smooth and cloudy-white and constricted. These worms may also wiggle or jump when disturbed, and can move across the ground in an S-shape like a snake. While the exact timing of their life cycle in MA might not be completely understood, their life cycle may be expected to go (roughly) something like this: they hatch in the late spring in 1-4 inches of soil, mature into adults during the summer and adults lay eggs sometime in August, and it is thought that their cocoons overwinter. (Adults perish with frost.) It is also worth noting here that jumping worms do not directly harm humans or pets.
For more information, listen to Dr. Olga Kostromytska’s presentation here: https://ag.umass.edu/landscape/education-events/invasive-insect-webinars
*NEW*: UMass Extension Fact Sheets:
Earthworms in Massachusetts – History, Concerns, and Benefits: https://ag.umass.edu/landscape/fact-sheets/earthworms-in-massachusetts-history-concerns-benefits
Jumping/Crazy/Snake Worms – Amynthas spp.:
https://ag.umass.edu/landscape/fact-sheets/jumpingcrazysnake-worms-amynthas-spp
Suggested reading includes Dr. Kostromytska’s recent “Hot Topics” article in Hort Notes (including an identification guide), here: https://ag.umass.edu/landscape/newsletters/hort-notes/hort-notes-2021-vol-323
Additional resources can also be found here:
University of Minnesota Extension: https://extension.umn.edu/identify-invasive-species/jumping-worms
Cornell Cooperative Extension: http://ulster.cce.cornell.edu/environment/invasive-pests/jumping-worm
UNH Extension: https://extension.unh.edu/blog/invasive-spotlight-jumping-worms
Tree & Shrub Insects & Mites:
- Asiatic Garden Beetle: Maladera castanea adults are active and are typically most abundant in July and August. These rusty-red colored beetles are bullet-shaped and active at night. They are often attracted to porch lights. They feed on a number of ornamental plants, defoliating leaves by giving the edges a ragged appearance and also feeding on blossoms. Butterfly bush, rose, dahlia, aster, and chrysanthemum can be favored hosts. Certain neem oil products are labelled for use against adult beetles. Observe label instructions to minimize the potential for leaf injury.

 Azalea Sawflies: There are a few species of sawflies that impact azaleas. Johnson and Lyon's Insects that Feed on Trees and Shrubs mentions three of them. Amauronematus azaleae was first reported in New Hampshire in 1895 and is likely found in most of New England. Adults of this species are black with some white markings and wasp-like. Generally green larvae feed mostly on mollis hybrid azaleas. Remember, sawfly caterpillars have at least enough abdominal prolegs to spell “sawfly” (so 6 or more prolegs). Adults are present in May, and females lay their eggs and then larvae hatch and feed through the end of June. There is one generation per year. Nematus lipovskyi has been reared from swamp azalea (Rhododendron viscosum). Adults of that species have been collected in April (in states to the south) and May (in New England) and larval feeding is predominantly in late April and May in Virginia and June in New England. One generation of this species occurs per year, and most mollis hybrid azaleas can be impacted. A third species, Arge clavicornis, is found as an adult in July and lays its eggs in leaf edges in rows. Larvae are present in August and September. Remember, Bacillus thuringiensis Kurstaki does not manage sawflies.
Azalea Sawflies: There are a few species of sawflies that impact azaleas. Johnson and Lyon's Insects that Feed on Trees and Shrubs mentions three of them. Amauronematus azaleae was first reported in New Hampshire in 1895 and is likely found in most of New England. Adults of this species are black with some white markings and wasp-like. Generally green larvae feed mostly on mollis hybrid azaleas. Remember, sawfly caterpillars have at least enough abdominal prolegs to spell “sawfly” (so 6 or more prolegs). Adults are present in May, and females lay their eggs and then larvae hatch and feed through the end of June. There is one generation per year. Nematus lipovskyi has been reared from swamp azalea (Rhododendron viscosum). Adults of that species have been collected in April (in states to the south) and May (in New England) and larval feeding is predominantly in late April and May in Virginia and June in New England. One generation of this species occurs per year, and most mollis hybrid azaleas can be impacted. A third species, Arge clavicornis, is found as an adult in July and lays its eggs in leaf edges in rows. Larvae are present in August and September. Remember, Bacillus thuringiensis Kurstaki does not manage sawflies. Bagworm: Thyridopteryx ephemeraeformis is a native species of moth whose larvae construct bag-like coverings over themselves with host plant leaves and twigs. In certain areas across MA in 2020, increased populations of bagworms were observed and reported, particularly in urban forest settings and managed landscapes. More information can be found here: https://ag.umass.edu/landscape/fact-sheets/bagworm
Bagworm: Thyridopteryx ephemeraeformis is a native species of moth whose larvae construct bag-like coverings over themselves with host plant leaves and twigs. In certain areas across MA in 2020, increased populations of bagworms were observed and reported, particularly in urban forest settings and managed landscapes. More information can be found here: https://ag.umass.edu/landscape/fact-sheets/bagworm
Bagworm caterpillars were reported defoliating honey locust in an urban forest setting in Northampton, MA on 7/27/2021. Caterpillars are quite large at this time, and feeding damage (defoliation of susceptible hosts) is apparent. These caterpillars develop into moths as adults. Their behaviors, life history, and appearance are interesting. The larvae (caterpillars) form “bags” or cases over themselves as they feed using assorted bits of plant foliage and debris tied together with silk. As the caterpillars feed and grow in size, so does their “bag”. Young, early instar caterpillars may feed with their bag oriented skyward, skeletonizing host plant leaves. As these caterpillars grow in size, they may dangle downward from their host plant, and if feeding on a deciduous host, they can consume the leaves down to the leaf veins. Pupation can occur in southern New England in late September or into October and this occurs within the “bag”. Typically, this means that the caterpillars could encounter a killing frost and die before mating could occur. However, in warmer areas of Massachusetts or if we experience a prolonged, warm autumn, it is possible for this insect to overwinter and again become a problem the following season. If the larvae survive to pupation, adult male moths emerge and are winged, able to fly to their flightless female mates. The adult male is blackish in color with transparent wings. The female is worm-like; she lacks eyes, wings, functional legs, or mouthparts. The female never gets the chance to leave the bag she constructs as a larva. The male finds her, mates, and the female moth develops eggs inside her abdomen. These eggs (500-1000) overwinter inside the deceased female, inside her bag, and can hatch roughly around mid-June in southern New England. Like other insects with flightless females, the young larvae can disperse by ballooning (spinning a silken thread and catching the wind to blow them onto a new host). While arborvitae and junipers can be some of the most commonly known host plants for this insect, the bagworm has a broad host range including both deciduous and coniferous hosts numbering over 120 different species. Bagworm has been observed on spruce, Canaan fir, honeylocust, oak, European hornbeam, rose, and London planetree among many others.
At this time, we are approaching the point where chemical management of bagworms may not be effective. This insect can be managed through physical removal, if they can be safely reached. Squeezing them within their bags or gathering them in a bucket full of soapy water (or to crush by some other means) can be effective ways to manage this insect on ornamental plants. Early instar bagworm caterpillars can be managed with Bacillus thuringiensis var. kurstaki (Btk) but this is most effective on young bagworms that are approximately no larger than ¾ inch in length. As bagworms grow in size, they may also have behavioral mechanisms for avoiding chemical management. At this point in the season, physical removal (if possible) may be the best option. This will also preserve any natural enemies that would be found attacking this insect, such as certain parasitic wasps. It is also important to note that the bags from dead bagworms will remain on the host plant, so check the viability of the bagworms by dissecting their bags to avoid unnecessary chemical applications. Historically in Massachusetts, bagworms have been mostly a problem coming in on infested nursery stock. With females laying 500-1000 eggs, if those eggs overwinter the population can grow quite large in a single season on an infested host. Typically this insect becomes a problem on hedgerows or plantings nearby an infested host plant. Thyridopteryx ephemeraeformis is found from Massachusetts to Florida, and is typically a more significant pest in southern climates. However, in recent years (2019-2021), bagworm appears to be overwintering in successfully in certain locations in Massachusetts.
- Dogwood Borer: Synanthedon scitula is a species of clearwing moth whose larvae bore not only into dogwood (Cornus), but hosts also include flowering cherry, chestnut, apple, mountain ash, hickory, pecan, willow, birch, bayberry, oak, hazel, myrtle, and others. Kousa dogwood appear to be resistant to this species. Signs include the sloughing of loose bark, brown frass, particularly near bark cracks and wounds, dead branches, and adventitious growth. The timing of adult emergence can be expected when dogwood flower petals are dropping and weigela begins to bloom. Adult moth flights continue from then until September. Emergence in some hosts (ex. apple) appears to be delayed, but this differs depending upon the location in this insect’s range. Eggs are laid singly, or in small groups, on smooth and rough bark. Female moths preferentially lay eggs near wounded bark. After hatch, larvae wander until they find a suitable entrance point into the bark. This includes wounds, scars, or branch crotches. This insect may also be found in twig galls caused by other insects or fungi. Larvae feed on phloem and cambium. Fully grown larvae are white with a light brown head and approx. ½ inch long. Pheromone traps and lures are useful for determining the timing of adult moth emergence and subsequent management.

 Dogwood Sawfly: Macremphytus tarsatus larvae are commonly seen feeding on dogwoods, especially gray dogwood (Cornus racemosa). One generation occurs per year. The larvae of the dogwood sawfly overwinter in decaying wood and occasionally (rarely) compromised structural timber. An overwintering “cell” is created in this soft wood. Pupation occurs in the springtime and adults can take a lengthy time to emerge, roughly from late May through July. 100+ eggs are laid in groups on the underside of leaves. Eggs hatch and the larvae feed gregariously, initially skeletonizing the leaves. As the caterpillars grow in size, they are capable of eating the entire leaf, leaving only midveins behind. Larval appearance varies greatly throughout instars. Early instars are translucent and yellow, but as the caterpillars grow, they develop black spots (over the yellow) and become covered in a white powder-like material. Larvae and their shed skins may resemble bird droppings. Full-grown larvae begin to wander in search of a suitable overwintering location. Rotting wood lying on the ground is preferred for this. Sawfly caterpillars can be collected from plants and dropped into a can of soapy water.
Dogwood Sawfly: Macremphytus tarsatus larvae are commonly seen feeding on dogwoods, especially gray dogwood (Cornus racemosa). One generation occurs per year. The larvae of the dogwood sawfly overwinter in decaying wood and occasionally (rarely) compromised structural timber. An overwintering “cell” is created in this soft wood. Pupation occurs in the springtime and adults can take a lengthy time to emerge, roughly from late May through July. 100+ eggs are laid in groups on the underside of leaves. Eggs hatch and the larvae feed gregariously, initially skeletonizing the leaves. As the caterpillars grow in size, they are capable of eating the entire leaf, leaving only midveins behind. Larval appearance varies greatly throughout instars. Early instars are translucent and yellow, but as the caterpillars grow, they develop black spots (over the yellow) and become covered in a white powder-like material. Larvae and their shed skins may resemble bird droppings. Full-grown larvae begin to wander in search of a suitable overwintering location. Rotting wood lying on the ground is preferred for this. Sawfly caterpillars can be collected from plants and dropped into a can of soapy water.
- Elongate Hemlock Scale: Fiorinia externa is found on eastern, Carolina, and Japanese hemlock, as well as yew, spruce, and fir. The elongate hemlock scale may overwinter in various life stages, and overlap of many developmental stages at any given time can be observed throughout much of the season. Nitrogen fertilizer applications may make elongate hemlock scale infestations worse.
- Exomala (Anomala) orientalis: is another introduced species (first detected in CT in 1920 from Japan) in the scarab beetle family (Scarabaeidae) that is typically smaller than the Japanese beetle as an adult. Adults are typically 4/10 of an inch, with the stout (broad) body typical of scarabs. Adult coloration can be variable, ranging from a black color to mottled gray with black patches/patterned markings. Exomala orientalis beetles can feed on flowers such as daisy, roses, phlox, and petunia as adults; although typically, the feeding by the adults is not severe. Larvae often coexist with those of the Japanese beetle and can be very difficult to distinguish from them. Larvae can damage the roots of turf grasses as well as the roots of many nursery plants and small fruits, including containerized plants. Common ornamental hosts include hemlock, holly, rhododendron, azalea, juniper, and andromeda. Adults are seen typically in mid-June and can be found through at least early August in MA. By late July, the larvae can cause serious damage to turfgrass and ornamental plants. The roots may be eaten and the crown girdled. This may lead to wilting and yellowing of foliage. Hand-pick adults when appropriate.
- Fall Home-Invading Insects: Various insects, such as ladybugs, boxelder bugs, seedbugs, and stink bugs will begin to seek overwintering shelters in warm places, such as homes, throughout the next couple of months. While such invaders do not cause any measurable structural damage, they can become a nuisance especially when they are present in large numbers. While the invasion has not yet begun, if you are not willing to share your home with such insects, now should be the time to repair torn window screens, repair gaps around windows and doors, and sure up any other gaps through which they might enter the home.
 Fall Webworm: Hyphantria cunea is native to North America and Mexico. It is now considered a world-wide pest, as it has spread throughout much of Europe and Asia. (For example, it was introduced accidentally into Hungary from North America in the 1940’s.) Hosts include nearly all shade, fruit, and ornamental trees except conifers. In the USA, at least 88 species of trees are hosts for these insects, while in Europe at least 230 species are impacted. In the past history of this pest, it was once thought that the fall webworm was a two-species complex. It is now thought that H. cunea has two color morphs – one black headed and one red headed. These two color forms differ not only in the coloration of the caterpillars and the adults, but also in their behaviors. Caterpillars may go through at least 11 molts, each stage occurring within a silken web they produce over the host. When alarmed, all caterpillars in the group will move in unison in jerking motions that may be a mechanism for self-defense. Depending upon the location and climate, 1-4 generations of fall webworm can occur per year. Fall webworm adult moths lay eggs on the underside of the leaves of host plants in the spring. These eggs hatch in late June or early July depending on climate. Young larvae feed together in groups on the undersides of leaves, first skeletonizing the leaf and then enveloping other leaves and eventually entire branches within their webs. Webs are typically found on the terminal ends of branches. All caterpillar activity occurs within this tent, which becomes filled with leaf fragments, cast skins, and frass. Fully grown larvae then wander from the webs and pupate in protected areas such as the leaf litter where they will remain for the winter. Adult fall webworm moths emerge the following spring/early summer to start the cycle over again. 50+ species of parasites and 36+ species of predators are known to attack fall webworm in North America. Fall webworms typically do not cause extensive damage to their hosts. Nests may be an aesthetic issue for some. If in reach, small fall webworm webs may be pruned out of trees and shrubs and destroyed. Do not set fire to H. cunea webs when they are still attached to the host plant.
Fall Webworm: Hyphantria cunea is native to North America and Mexico. It is now considered a world-wide pest, as it has spread throughout much of Europe and Asia. (For example, it was introduced accidentally into Hungary from North America in the 1940’s.) Hosts include nearly all shade, fruit, and ornamental trees except conifers. In the USA, at least 88 species of trees are hosts for these insects, while in Europe at least 230 species are impacted. In the past history of this pest, it was once thought that the fall webworm was a two-species complex. It is now thought that H. cunea has two color morphs – one black headed and one red headed. These two color forms differ not only in the coloration of the caterpillars and the adults, but also in their behaviors. Caterpillars may go through at least 11 molts, each stage occurring within a silken web they produce over the host. When alarmed, all caterpillars in the group will move in unison in jerking motions that may be a mechanism for self-defense. Depending upon the location and climate, 1-4 generations of fall webworm can occur per year. Fall webworm adult moths lay eggs on the underside of the leaves of host plants in the spring. These eggs hatch in late June or early July depending on climate. Young larvae feed together in groups on the undersides of leaves, first skeletonizing the leaf and then enveloping other leaves and eventually entire branches within their webs. Webs are typically found on the terminal ends of branches. All caterpillar activity occurs within this tent, which becomes filled with leaf fragments, cast skins, and frass. Fully grown larvae then wander from the webs and pupate in protected areas such as the leaf litter where they will remain for the winter. Adult fall webworm moths emerge the following spring/early summer to start the cycle over again. 50+ species of parasites and 36+ species of predators are known to attack fall webworm in North America. Fall webworms typically do not cause extensive damage to their hosts. Nests may be an aesthetic issue for some. If in reach, small fall webworm webs may be pruned out of trees and shrubs and destroyed. Do not set fire to H. cunea webs when they are still attached to the host plant.- Hibiscus Sawfly: The larvae of the hibiscus (mallow) sawfly, likely Atomacera decepta, may be observed feeding on hibiscus hosts at this time. Sawfly larvae develop into wasp-like adults (Order: Hymenoptera) and therefore these “caterpillars” will not be managed by Bacillus thuringiensis var. kurstaki which is specific to the Lepidoptera (caterpillars that develop into moths or butterflies as adults). Reduced risk active ingredients such as spinosad are labelled for use against sawfly larvae. However, given that hibiscus are very attractive to pollinators, non-chemical management options such as hand picking and disposing of larvae, when possible, are best. Spinosad is toxic to pollinators until it dries. For more information about the risks of insecticide active ingredients to pollinators, visit: https://ag.umass.edu/landscape/fact-sheets/tree-shrub-insecticide-active-ingredients-risks-to-pollinators-other-non .
The hibiscus (mallow) sawfly adult female uses her ovipositor to cut slits into leaf surfaces to deposit her eggs. Larvae emerge from these eggs and begin by first feeding on leaf undersides when small, and then move to feed on leaf surfaces as they grow in size. Only large leaf veins may be left behind if the population is large enough. Larvae have been observed moving to the base of the plant to pupate. Adults emerge and in some locations in the US, multiple generations have been recorded per year. This insect is known in the mid-Atlantic and Midwest states, but was reported feeding on Hibiscus spp. in Connecticut in 2004 and 2005 and has previously been reported in Massachusetts. The timing of the life cycle of this insect, as well as how many generations occur per year in Massachusetts, however, is not fully understood. Some research has shown that Hibiscus acetosella, H. aculeatus, and H. grandiflora seem to either exhibit some resistance to or tolerance of hibiscus sawfly feeding. In one study, all three had few if any eggs or larvae and were given the lowest damage rating among the species evaluated. This insect also does not feed on rose of Sharon or H. rosasinensis. It has, however, been reported to “voraciously” feed on H. moscheutos, H. palustris, H. militaris, and H. lasiocarpus.
- Hickory Tussock Moth: Lophocampa caryae is native to southern Canada and the northeastern United States. There is one generation per year. Overwintering occurs as a pupa inside a fuzzy, oval shaped cocoon. Adult moths emerge approximately in May and their presence can continue into July. Females will lay clusters of 100+ eggs together on the underside of leaves. Females of this species can fly, however they have been called weak fliers due to their large size. When first hatched from their eggs, the young caterpillars will feed gregariously in a group, eventually dispersing and heading out on their own to forage. Caterpillar maturity can take up to three months and color changes occur during this time. These caterpillars are essentially white with some black markings and a black head capsule. They are very hairy, and should not be handled with bare hands as many can have skin irritations or rashes (dermatitis) as a result of interacting with hickory tussock moth hairs. By late September, the caterpillars will create their oval, fuzzy cocoons hidden in the leaf litter where they will again overwinter. Hosts whose leaves are fed upon by these caterpillars include but are not limited to hickory, walnut, butternut, linden, apple, basswood, birch, elm, black locust, and aspen. Maple and oak have also been reportedly fed upon by this insect. Several wasp species are parasitoids of hickory tussock moth caterpillars.
- Lacebugs: Stephanitis spp. lacebugs such as S. pyriodes can cause severe injury to azalea foliage. S. rhododendri can be common on rhododendron and mountain laurel. S. takeyai has been found developing on Japanese andromeda, leucothoe, styrax, and willow. Stephanitis spp.lace bug activity should be monitored through September. Before populations become too large, treat with a summer rate horticultural oil spray as needed. Be sure to target the undersides of the foliage in order to get proper coverage of the insects. Certain azalea and andromeda cultivars may be less preferred by lace bugs.
 Magnolia Scale: Neolecanium cornuparvum is a soft scale that overwinters as first instar nymphs which are elliptical, and dark slate gray in color and can usually be found on the undersides of 1 and 2 year old twigs. Nymphs may molt by late April or May and again by early June at which time the scales may be purple in color. Eventually nymphs secrete a white powdery layer of wax over their bodies, looking as if they have been rolled in powdered sugar. By August, the adult female scale is fully developed, elliptical and convex in shape and ranging from a pinkish-orange to a dark brown in color. Adult females may also be covered in a white, waxy coating. By that time, the females produce nymphs (crawlers; living young; eggs are not “laid”) that wander the host before settling on the newest twigs to overwinter. In the Northeastern United States, this scale insect has a single generation per year.
Magnolia Scale: Neolecanium cornuparvum is a soft scale that overwinters as first instar nymphs which are elliptical, and dark slate gray in color and can usually be found on the undersides of 1 and 2 year old twigs. Nymphs may molt by late April or May and again by early June at which time the scales may be purple in color. Eventually nymphs secrete a white powdery layer of wax over their bodies, looking as if they have been rolled in powdered sugar. By August, the adult female scale is fully developed, elliptical and convex in shape and ranging from a pinkish-orange to a dark brown in color. Adult females may also be covered in a white, waxy coating. By that time, the females produce nymphs (crawlers; living young; eggs are not “laid”) that wander the host before settling on the newest twigs to overwinter. In the Northeastern United States, this scale insect has a single generation per year.
On magnolia hosts, these large soft scales could either be Neolecanium cornuparvum, the magnolia scale, or Toumeyella liriodendri, the tuliptree scale. The tuliptree scale can be found both on magnolia and tuliptree hosts, whereas the magnolia scale is only known to magnolia. Differentiation between these two species of scale (on magnolia) can be difficult in the field.
- Tuliptree Aphid: Illinoia liriodendri is a species of aphid associated with the tuliptree, wherever it is grown. (They may at times also feed on magnolia, according to reports.) Depending upon local temperatures, these aphids may be present from mid-June through early fall. Large populations can develop by late summer. Some leaves, especially those in the outer canopy, may brown and drop from infested trees prematurely. The most significant impact these aphids can have is typically the resulting honeydew, or sugary excrement, which may be present in excessive amounts and coat leaves and branches, leading to sooty mold growth. This honeydew may also make a mess of anything beneath the tree. Wingless adults are approximately 1/8 inch in length, oval, and can range in color from pale green to yellow. There are several generations per year. This is a native insect. If management is deemed necessary, select options that will preserve natural enemies, as ladybeetles and other beneficial insects are often associated with the tuliptree aphid.
- Twolined Chestnut Borer: Agrilus bilineatus is a native jewel beetle (also known as a flatheaded borer) in the Family Buprestidae. This insect is also in the same genus as the invasive emerald ash borer. The twolined chestnut borer is native to Massachusetts, much of New England, and the eastern United States. This species has one generation per year and adults are typically active from April – August, depending upon location and temperature. Adults will conduct some maturation feeding on oak prior to mating. Females will lay clusters of tiny eggs in the cracks and crevices of bark. Larvae hatch from the eggs in 1-2 weeks and burrow through the bark into the cambium, where they feed in a similar manner to the emerald ash borer, creating meandering galleries as they feed. (The galleries of the twolined chestnut borer can be straight in very stressed trees.) Larvae typically mature by August – October and burrow to the outer bark where they create a chamber in which they overwinter. Pupation occurs the following spring and adults emerge through D-shaped exit holes that are approximately 1/5 inch wide. In the northern extent of this insect’s range, they can take 2 years to complete their life cycle. Larvae of this insect have been recorded from eastern white oak, common post oak, burr oak, scarlet oak, northern red oak, and eastern black oak. Adults have been recorded on fir and pin oak. These insects are attracted to stressed host plants and typically become a secondary factor in the decline of the tree.
- Two-Spotted Spider Mite: Tetranychus urticae is a “warm-season” mite that loves hot and dry weather, which may favor the quick reproduction and build-up of this pest. Management should seek to preserve beneficial predatory mites. Monitor susceptible hosts (elm, maple, redbud, ash, black locust, tuliptree, and many deciduous shrubs) for increasing numbers of these mites until mid-August. Mites will be found on the undersides of leaves and cause stippling of the foliage.
- Viburnum Leaf Beetle: Pyrrhalta viburni is a beetle in the family Chrysomelidae that is native to Europe, but was found in Massachusetts in 2004. This beetle feeds exclusively on many different species of viburnum, which includes, but is not limited to, susceptible plants such as V. dentatum, V. nudum, V. opulus, V. propinquum, and V. rafinesquianum. Some viburnum have been observed to have varying levels of resistance to this insect, including but not limited to V. bodnantense, V. carlesii, V. davidii, V. plicatum, V. rhytidophyllum, V. setigerum, and V. sieboldii. More information about viburnum leaf beetle may be found at http://www.hort.cornell.edu/vlb/ .






 White Satin Moth: Leucoma salicis was recently reported from Beartown State Forest (Berkshire County) on 6/23/2021 by the Massachusetts Department of Conservation and Recreation, Forest Health Program. This is the same location that caterpillars of this species were seen defoliating their hosts in 2020. Caterpillars, pupae, adult moths, and egg masses were observed at this location on 6/24/2021. Very noticeable predation of the adult moths by birds was also witnessed, along with the aftermath of this predation (remains of white satin moth wings on the ground).
White Satin Moth: Leucoma salicis was recently reported from Beartown State Forest (Berkshire County) on 6/23/2021 by the Massachusetts Department of Conservation and Recreation, Forest Health Program. This is the same location that caterpillars of this species were seen defoliating their hosts in 2020. Caterpillars, pupae, adult moths, and egg masses were observed at this location on 6/24/2021. Very noticeable predation of the adult moths by birds was also witnessed, along with the aftermath of this predation (remains of white satin moth wings on the ground).
The caterpillars of this species have a unique color pattern, which helps us distinguish them from others. The dorsal (back) side of the caterpillar is marked with 10-11 white, intersegmental spots as well as paired, red “setal warts”. The sides of the caterpillars are blueish gray. These caterpillars are known to the edges of waterways, woodlands, and forests from Canada to northwestern Connecticut and central New York. One generation occurs per year with mature caterpillars known in May and June. Host plants include aspen, poplar, and willow and are fed upon by the caterpillars of this species.
The white satin moth was introduced from Europe and first reported between Boston, MA and Hampton, New Hampshire in 1920. This insect is said to overwinter in the third instar (caterpillars pass through seven instars), either individually or in small groups. In the spring time, caterpillars leave their areas of hibernation to feed on nearby leaves. Caterpillars spin a thin cocoon between leaves or between exfoliating or thick bark crevices. Pupae are dark brown/black and often in a thin, loose silken sack. Pupae also sport brightly colored, yellow setae (hairs) that make them quite attractive. Pupation begins by the end of June. Shortly thereafter, moths emerge and females lay egg masses covered in a frothy, white material from July – mid-August. Eggs hatch sometime in August, and larvae will conduct feeding in August and September.
While caterpillars of this species are not noted to be of particular concern with regard to causing allergic reactions such as dermatitis, they are a type of tussock moth and do possess hairs, so they should not be handled/approached with caution particularly by sensitive individuals.
Concerned that you may have found an invasive insect or suspicious damage caused by one? Need to report a pest sighting? If so, please visit the Massachusetts Introduced Pests Outreach Project: http://massnrc.org/pests/pestreports.htm .
Reported by Tawny Simisky, Extension Entomologist, UMass Extension Landscape, Nursery, & Urban Forestry Program
Additional Resources
Pesticide License Exams - The MA Dept. of Agricultural Resources (MDAR) is now holding exams online. For more information and how to register, go to: https://www.mass.gov/pesticide-examination-and-licensing.
To receive immediate notification when the next Landscape Message update is posted, join our e-mail list or follow us on Facebook.
For a complete listing of upcoming events, see our upcoming educational events https://ag.umass.edu/landscape/upcoming-events
For commercial growers of greenhouse crops and flowers - Check out UMass Extension's Greenhouse Update website
For professional turf managers - Check out Turf Management Updates
For home gardeners and garden retailers - Check out our home lawn and garden resources.
Diagnostic Services
UMass Laboratory Diagnoses Landscape and Turf Problems - The UMass Extension Plant Diagnostic Lab is available to serve commercial landscape contractors, turf managers, arborists, nurseries and other green industry professionals. It provides woody plant and turf disease analysis, woody plant and turf insect identification, turfgrass identification, weed identification, and offers a report of pest management strategies that are research based, economically sound and environmentally appropriate for the situation. Accurate diagnosis for a turf or landscape problem can often eliminate or reduce the need for pesticide use. For sampling procedures, detailed submission instructions and a list of fees, see Plant Diagnostic Laboratory
Soil and Plant Nutrient Testing - The University of Massachusetts Soil and Plant Nutrient Testing Laboratory is located on the campus of The University of Massachusetts at Amherst. Testing services are available to all. The lab provides test results and recommendations that lead to the wise and economical use of soils and soil amendments. For more information, visit the UMass Soil and Plant Nutrient Testing Laboratory web site. Routine soil analysis and particle size analysis ONLY (no other types of soil analyses available at this time). Turnaround time: Please plan for the fact that date of receipt in the lab is affected by weekends, holidays, shipping time, and time for UMass Campus Mail to deliver samples to the lab. Campus Mail delivery only takes place on Monday, Wednesday, and Friday due to pandemic restrictions.
Tick Testing - The UMass Center for Agriculture, Food, and the Environment provides a list of potential tick identification and testing options at: https://ag.umass.edu/resources/tick-testing-resources.